Quick Study Analysis: The Fat-1 Mouse in Obesity
"All models are wrong, but some are useful."—George Box
“Omega-3 Polyunsaturated Fatty Acids Protect against High-Fat Diet-Induced Morphological and Functional Impairments of Brown Fat in Transgenic Fat-1 Mice”
Of the many problems with nutritional animal research, one of the most annoying is the difficulty in composing diets that actually represent what the researcher is trying to study.
In studying the effects of fats on an organism, the hoped-for mix of fats is often targeted by blending various fats and oils together to get the desired input, often with varying degrees of success. Additionally, natural fats often contain a range of ‘contaminants’ that might affect the outcome of the study.
This paper (Hao 2022) uses a different approach. In 2004 the senior author Jing Kang developed a transgenic mouse to allow for a cleaner approach to studying the effects of Ω-3 and -6 fats in various diseases (Kang, 2004; 2008).
Rather than blend various fats to hopefully achieve the desired diet, he changed the mice.
Mammals, and most higher animals, are unable to convert Ω-6 fats to Ω-3 fats. Hence we have a dietary requirement for each type of fat—they are ‘essential’ in the term of art.
However, C. elegans, the roundworm often used in research due to its simplicity and ease of study, can convert Ω-6 to -3.
Kang created a mouse that includes the fat-1 gene from C. elegans, which allows them to convert dietary Ω-6 into Ω-3. Specifically, it seems, they convert linoleic acid (LA) to alpha-linolenic acid (ALA) the base Ω-3 chain from which the actually-essential long-chain Ω-3 fats like EPA and DHA may be created. And since these mice are converting these fats, this modification simultaneously reduces the Ω-6 fats in tissues, resulting in a 1:1 ratio of the two types of fats (approximately).
Thus a study can have a ‘wild-type’ (WT) mouse without the mutation and a fat-1 mouse with the mutation, both eating exactly the same diet.
What have we learned from fat-1?
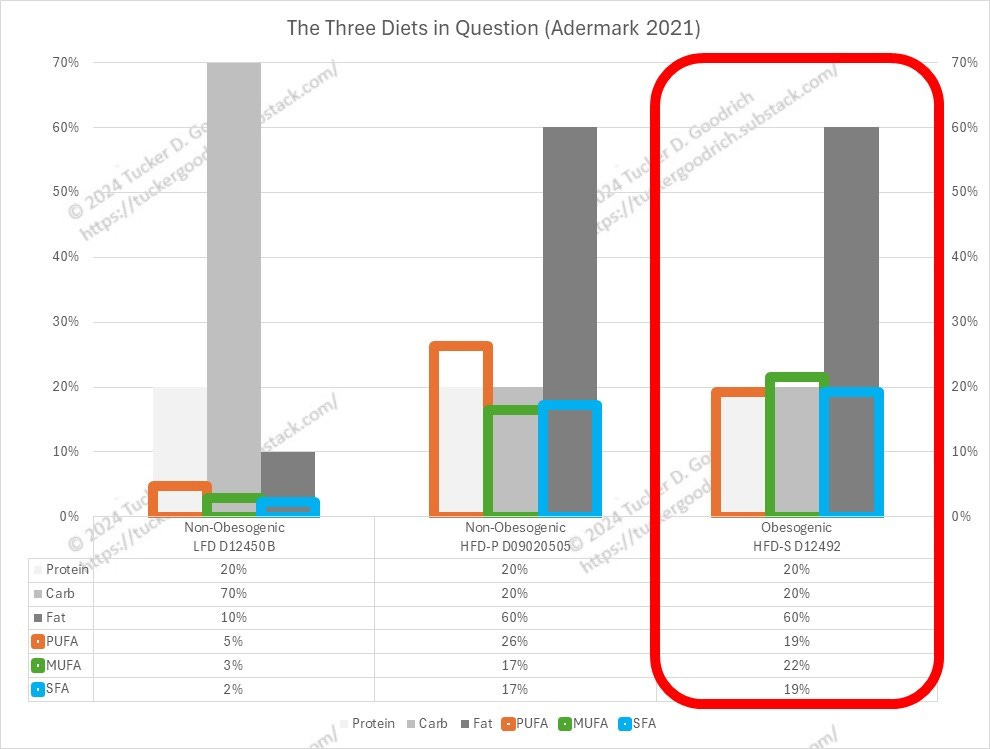
Kang in this 2022 paper compares a high-sucrose, low-fat diet (TestDiet 1812692) to my favorite, Research Diets’ D12492, discussed at length in this post:
Does Linoleic Acid Induce Obesity? Part 1
[P.S. Part 2: Roux-en-Y Gastric Bypass and 4-Hydroxynonenal.] Introduction OK folks, into the wayback machine.Thanks for reading Tucker Goodrich: yelling Stop! Subscribe for free to receive new posts and support my work. In 2010 I stopped eating seed oils, and saw a chronic inflammatory bowel disease resolve in days:
D12492 is the most-used diet in laboratories to induce obesity, and is similar in composition the the human diet commonly used to help patients gain weight, as well as the fat composition of the Modern American Diet.
And as one familiar with the effects of omega PUFAs would expect, the fat-1 mice exhibit a degree of protection from the obesogenic effects of this diet.
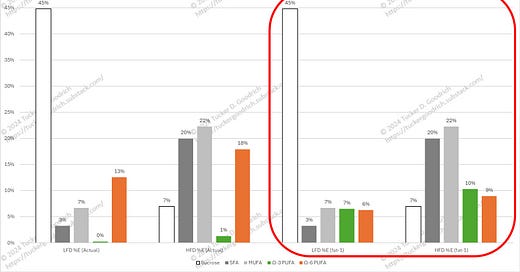
The effect of the diet is less obesogenic in the fat-1 mouse than the WT mice. It’s not surprising that they’re not entirely protected, as the diet is very high in Ω-6 PUFA to start with. To make this clear, I created this graph:
The two groups of bars on the left represent what these mice actually ate, and the two sets on the right (red box) represent what the fat-1 mouse may have essentially be eating. Fifty percent of the Ω-6 in converted to Ω-3 in the fat-1 diets. Is this what is actually happening in these mice? See outstanding questions at the end.
(I included the levels of sugar (sucrose—white bars) in these diets since I thought it might amuse Gary Taubes to see that the level of sucrose (45% vs. 7%) is inversely related to the obesogenic effect of these diets, and that reducing this level is not required to ameliorate the effect. Seriously, as a follow-up to my post “Quick Study Analysis: The Fat Curve, part 1” it is interesting that LA of 18% with additional fat is so much more fattening than LA of 13% with 45% sucrose.)
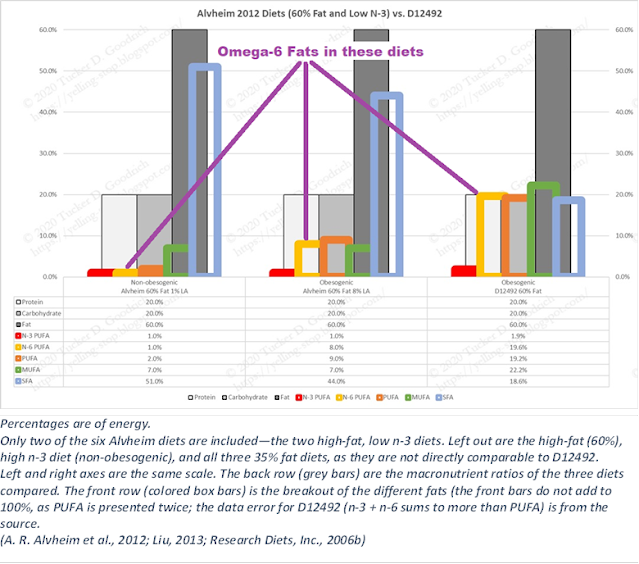
But what we can see is that after conversion the level of fat these mice are eating is down to the level of the obesogenic diets in (Alvheim 2012).
They’re still getting an obesogenic dose of Ω-6, and even the 10% of converted (?) Ω-3 is not enough to counter this.
In episode 22 of the podcast I discussed fats and obesity with John Speakman:
Ep. 22: John Speakman—What Causes Obesity?
Introduction I am honored to be joined by John Speakman, a leading authority on energy balance in animals, and in the use of the doubly-labeled water method of determining energy balance. This has led him into the study of obesity, among other topics, where he has become a recognized expert.
This conversation was spurred by his finding that the metabolic rate of humans has declined, and his question about how Ω-6 fats could be involved in this.
Hao et al. found that the HFD impaired metabolism and the functioning of brown adipose tissue, which is part of the temperature-regulating system of mice and humans.
And that the fat-1 mice were protected from this effect.
“A novel finding in our study is that HFD feeding led to “whitening” in brown fat tissue in WT mice, whereas fat-1 mice were protected against such morphology change caused by HFD feeding.” (Hao, 2022)
Conclusion
As this is getting long enough, they found that many aspects of the metabolic-syndrome were improved by endogenously converting Ω-6 to -3 fats, including glucose tolerance and metabolic rate, as well as a better inflammatory response.
There are many other papers looking at varying aspects of human disease using these mice, some with more success than others, but that’s outside the scope of this post.
This is clearly a useful and interesting model, and it implicates excess Ω-6 fats in a range of human pathologies.
I do have a couple of questions about the fat-1 mouse:
Where is this conversion taking place?
It’s a systemic update, but the data I have seen is looking primarily in what is known as “invisible fat”, the fats in cell membranes. While milk is affected, milk is comprised of fats produced or converted in the mother’s body. I would expect lipoprotein payloads of fats and adipose stores of fats would more closely resemble the diet, as I would be surprised if the mouse is converting fats as they are being digested, although this is a possibility.What’s the genetic background of the fat-1 mouse?
’Wild-type’ typically doesn’t really mean that, instead it’s some laboratory-derived strain bred because is is susceptible to some disease like obesity.
I’ll continue to look into the research that has been generated from this model. “More study required”, indeed!
P.S. Why the interest in the fat-1 mouse?
“Amelioration of UVB-Induced Oxidative Stress and Inflammation in Fat-1 Transgenic Mouse Skin.”
Quick Post on Sunburn and Seed Oils
Ultra-violet radiation causes Ω-6 fats to autoxidize to 4-HNE, promoting ferroptosis and the inflammatory cascade typical of sunburn. Blocking ferroptosis topically on skin reduces production of 4-HNE: “Keratinocyte Death by Ferroptosis Initiates Skin Inflammation After UVB Exposure” (Vats, 2021)
References
Alvheim, A. R., Malde, M. K., Osei‐Hyiaman, D., Hong, Y. H., Pawlosky, R. J., Madsen, L., Kristiansen, K., Frøyland, L., & Hibbeln, J. R. (2012). Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity, 20(10), 1984–1994. https://doi.org/10.1038/oby.2012.38
Goodrich, T. D. (2021, November 18). Does Linoleic Acid Induce Obesity? Part 1 [Blog]. Yelling Stop. https://tuckergoodrich.substack.com/p/does-linoleic-acid-induce-obesity
Goodrich, T. D. (2024, May 23). Quick Study Analysis: The Fat Curve, part 1 [Substack newsletter]. Tucker Goodrich: Yelling Stop. https://tuckergoodrich.substack.com/p/quick-study-analysis-the-fat-curve
Hao, L., Nie, Y.-H., Chen, C.-Y., Li, X.-Y., Kaliannan, K., & Kang, J. X. (2022). Omega-3 Polyunsaturated Fatty Acids Protect against High-Fat Diet-Induced Morphological and Functional Impairments of Brown Fat in Transgenic Fat-1 Mice. International Journal of Molecular Sciences, 23(19), Article 19. https://doi.org/10.3390/ijms231911903
Kang, J. X., Wang, J., Wu, L., & Kang, Z. B. (2004). Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature, 427(6974), 504. https://doi.org/10.1038/427504a
Kang, J. X. (2008). A Transgenic Mouse Model for Gene-Nutrient Interactions. Journal of Nutrigenetics and Nutrigenomics, 1(4), 172–177. https://doi.org/10.1159/000119714
Rumora, A. E., LoGrasso, G., Hayes, J. M., Mendelson, F. E., Tabbey, M. A., Haidar, J. A., Lentz, S. I., & Feldman, E. L. (2019). The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. The Journal of Neuroscience, 39(19), 3770–3781. https://doi.org/10.1523/JNEUROSCI.3173-18.2019












This was very interesting. Thank you for writing this.
Was this the study referenced in Omega Balance with a similar result? That was by far the #1 most curious factoid in that book, I thought, speaking for the o6 theory. If we can essentially "suck out" the o6 entering from the diet and everything gets healthier..