[P.S. Part 2: Roux-en-Y Gastric Bypass and 4-Hydroxynonenal.]
Introduction
OK folks, into the wayback machine.
In 2010 I stopped eating seed oils, and saw a chronic inflammatory bowel disease resolve in days:
“So a few months ago, I stopped eating industrial seed oils (veggie oils). In two days the diarrhea stopped. Eat the oils, it started again. I no longer craved starch or sugar, so I didn't eat any wheat for a week, without meaning to…. So now I could turn the symptoms of the last 16 years on or off, based on eating veggie oils or wheat. Wow!” (Goodrich, 2010)
This was spurred in large part by the writings of Stephan Guyenet, to whom I owe a debt for his work. As I emailed Stephan at the time:
“One of the first things I noticed after dropping n-6 from my diet was that I was no longer craving starch and sugar…. It makes me wonder if there might be a mechanism linking the two..." (Goodrich, 2011)
The lack of desire (cravings) for carbohydrate enabled me to go on a low-carb diet for the first time (I had tried before, and failed due to the cravings), and within the next two months I lost my excess weight. I’ve been weight-stable since then, without trying—no counting of calories—although I am strict about staying on the diet.
What I had done is something that apparently no-one else had done: stop eating seed oils as my first step in changing my diet. And it had a profound effect on my weight, eating habits, and health.
So imagine my surprise when Stephan posted this:
“As my knowledge of obesity and metabolism has expanded, I feel the evidence behind the hypothesis that seed oils (corn, soybean, etc.) promote obesity due to their linoleic acid (omega-6 fat) content has largely collapsed.” (S. J. Guyenet, 2011a)
I was flabbergasted. Nevertheless, the fact that Stephan had changed his mind didn’t affect my opinion, which was based largely on my own rather dramatic experiences. I continued to look into the topic.
The following month Stephan posted about his new interest, as his career moved forward:
“Just as in rats, exposing humans to a variety of readily accessible, energy dense, palatable foods causes excessive food intake and rapid weight gain. The degree of overeating varies by individual, but nearly everyone overeats to some degree. Whatever the mechanism(s) underlying this may be, the phenomenon has important implications for the commercialization of food and the associated obesity epidemic in affluent nations.” (S. J. Guyenet, 2011b)
Stephan went on:
“High fat diets, particularly in combination with refined starches and sugars, were among the most effective. The composition of these diets has been refined since then, and modern "purified" high-fat diets [HFD] reliably induce obesity in susceptible strains of rodents. The most commonly used diet is Research Diets D12492, [ (Research Diets, Inc., 2006b) ] which is 60% fat by calories, and composed mostly of lard, soybean oil, casein, maltodextrin, sucrose and cellulose (7). It tastes kind of like raw cookie dough, and the rats are crazy about it.”
As Research diets stated in 2009, “Our DIO [diet-induced obesity] series diets D12450B, D12451, and D12492 are the most widely used and published high fat diets worldwide” (Research Diets, Inc., 2009).
The ”cookie dough” statement became somewhat notorious in certain circles (Dobromylskyj, 2012), who found the notions of palatability and reward to be too vague.
This became known as the “Food Reward Hypothesis”, (S. J. Guyenet, 2011c), in which the trait of “palatability” causes animals (including humans) to over eat. These foods are known as hyper-palatable foods, those which induce over-consumption and weight gain.
“Palatability refers specifically to the enjoyment derived from a food, also called its hedonic value. Palatability and reward typically travel together, but not always.” (S. J. Guyenet, 2011c)
And as Stephan notes, this seems to be mediated in the brain, although as he also noted, the mechanism(s) were not known. He further explored this idea in a formal study, “Regulation of Food Intake, Energy Balance, and Body Fat Mass: Implications for the Pathogenesis and Treatment of Obesity” (S. J. Guyenet & Schwartz, 2012).
Linoleic Acid and Obesity
A Revelation About D12492
My second quote above, the email to Stephan, was from a post I did in 2011, titled “Linoleic Acid, Fat Rats in Labs, and Fat Humans.” (Goodrich, 2011)
Chris Masterjohn (a friend and colleague of Stephan’s) had posted about D12492, the cookie-dough diet. As detailed in my post, the manufacturer had disclosed to Chris that they discovered the composition of the diet wasn’t what they had thought:
"...I got an email today from Dr. Matthew Ricci, the Vice-President and Research Director of Research Diets, the company that produces the infamous 60% fat, lard-based rodent diet D12492. I've written about this diet before. The company had previously been using the USDA database to determine the diet's fatty acid profile, but recently had it directly analyzed, knowing that the fatty acid profile of lard can vary according to what the pigs are fed.
"It turns out that the diet obtains 32% of its fat from PUFA instead of the previously reported 17%. The ratio of omega-6 linoleic acid to omega-3 linolenic acid had been previously reported as 7.8 but is actually 14...."
I observed:
“I was very surprised when [Stephan] posted [ (S. J. Guyenet, 2011a) ], as his previous position coincided perfectly with my experience as stated above. It also turns out that the research he now quotes in support of the problematic food reward hypothesis also supports the position he's abandoned, given this updated information on the composition of the diets he cites.
“Stephan may have reconsidered his position on seed oils, but I haven't. I still avoid them like the plague, as they had a clear effect on me once I stopped eating them.”
However, there wasn’t great evidence—that I was aware of at the time—for the hypothesis that linoleic acid (LA, aka seed oils, omega-6 fats, ω-6, or n-6) caused obesity. The fact that it was in the cookie-dough diet, and that there was more than was known in the cookie dough diet, didn’t mean that n-6 caused the obesity that the diet caused.
I’ve communicated with Stephan since then, and he explained that after that 2011 post, he dropped the topic of linoleic acid and obesity, and moved on. That’s fair enough, I’ve abandoned some seemingly-unfruitful hypotheses. He said he’d reconsider in light of new evidence, so that’s what I’m looking to provide.
“Dietary Linoleic Acid… Induces Obesity”
That didn’t take long.
In 2012 a group led by Joseph Hibbeln released a paper titled, “Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity”. (A. R. Alvheim et al., 2012). Hibbeln is a renowned researcher at the NIH (NIH, 2018), and had in fact provided the data for Stephan’s later paper looking at the rate of increase of LA in adipose tissue in humans in the 20th century. (S. J. Guyenet & Carlson, 2015)
Alvheim looked directly at the issue, for the first time, and found (as the title suggests), that n-6 fats do induce obesity in a dose-dependent manner, although with a confounder.
“These ecological associations do not demonstrate that increasing LA caused increasing obesity in the United States during the 20th century. However, the results of the causal test of this inference in rodents are consistent with the interpretation that increasing dietary LA in human diets… substantially contributed to increasing prevalence rates of obesity.”
Alvheim fed mice a diet containing either 1% LA, 8% LA, or 8% LA + 1% omega-3 fats:
“Here we demonstrate that… obesity can be caused by elevating a single molecular species in the diet, LA, an ω-6 essential fatty acid…
“We modeled human dietary increases in LA from 1 en% to 8 en% which significantly increased LA-PL [linoleic acid in phospholipids], AA-PL [arachidonic acid phospholipids] in liver and erythrocytes, nearly tripled liver 2-AG and AEA, resulting in increased food intake, feed efficiency, plasma leptin, and adiposity. Decreasing AA-PL in liver and erythrocyte by adding 1 en% from EPA and DHA reduced 2-AG + 1-AG and AEA levels, reduced feed efficiency and reversed the adipogenic effects of the 8 en% LA diets.”
The confounder is their observation that n-3 (omega-3) fats were protective from the obesity-inducing effect of n-6.
I’ve compared the Alvheim diets to the obesogenic D12492 (cookie-dough) diet:
The left diet is non-obesogenic, and the other two are obesogenic. The two Alvheim diets were constructed so that, “LA was isolated as an independent variable by mixing seven different oils to maintain equivalent amounts of α-LA and monounsaturates [MUFA]…” (Fatty-acid composition for D12492 is from (Liu, 2013))
Going from LA of 1% of energy to 8% changes a non-obesogenic diet to an obesogenic one. Then we note that the highly obesogenic D12492 diet has nearly 20% of energy as LA.
Alvheim chose the 8% LA quantity to reflect changes in the human diet over the 20th century, but one can’t help but think that they had comparisons to lab diets such as D12492 in mind, although they didn’t mention it.
(A note on these results: the standard model of obesity in terms of fat consumption attributes the effect of the high-fat diet (such as D12492) to saturated fats (SFA). These results clearly prove that is wrong, as the non-obesogenic diet has 51% of calories from SFA. Next up is the hypothesis that monounsaturated fats (MUFA) might be protective, but here the higher MUFA and lower SFA in D12492 offer no protection from obesity as compared to the 51% SFA non-obesogenic Alvheim diet, as literally countless experiments in the literature have demonstrated.)
But before you simply conclude that the food reward hypothesis is wrong, please keep reading, as it’s not that simple. Alvheim et al. described a mechanism, which we will discuss below.
One Study Is Useless, We Need Replication
The definition of the word “science” is “knowledge”, but more specifically it’s “reliable knowledge”. It must consistently produce the same result, or it is not “reliable”. So in order for a scientific study to be considered reliable, it must be replicated, ideally a lot. Then we know that the result is reliable, as it is repeatable.
Anita Alvheim went on to do several more studies on this topic, some with Joseph Hibbeln, some with Norwegian researchers. In order of publication, they are:
“Intake of farmed Atlantic salmon fed soybean oil increases insulin resistance and hepatic lipid accumulation in mice” (Midtbø et al., 2013)
“Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice” (A. R. Alvheim et al., 2013)
“Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet” (A. Alvheim et al., 2014)
The first two of those studies are looking at the effects of feeding soybean oil (basically) to salmon, and then observing the effects of feeding those salmon to mice. They were performed contemporaneously, and produce slightly different results, although both show harm from the high n-6 diet, although the first doesn’t demonstrate weight gain. This is outside the scope of this article, but appears to be consistent with (A. R. Alvheim et al., 2012) above, where n-3 fats were protective. The amounts of these fats in relation to each other do seem to matter.
The third study (A. Alvheim et al., 2014) is basically a follow-up to (A. R. Alvheim et al., 2012), as they add a low-fat [LFD] arm with a comparison to a 35% fat arm like that used in the previous study.
“We find that the LFD containing 8 en% [LA] significantly increased feed efficiency compared to the 1 en% [LA] diet causing the animals to gain more weight per calorie consumed. The increased feed efficiency and weight gain in mice fed 8 en% [LA] in the LFD resulted in a similar body weight as mice fed 35 en% fat, significantly higher than mice fed 1 en% [LA] in an LFD. Dietary [LA] of 1 en% has been found to reverse the obesogenic properties of a high fat diet [14]. In line with previous studies, our findings in the LFD support the notion that weight gain involves mechanisms that depend more on the composition of dietary fat than the total amount of fat in the diet [12, 30, 41–43].”
While some interesting confirmatory looks at this line of work has been done concerning mechanisms, on which more later, direct confirmation took a while.
We have to wait until 2019 for a confirmation (or at least, I couldn’t find one that was earlier). (Ghosh et al., 2019) Ghosh replicated the basic finding, and added glucose to look at what the combined effect would be (not good, in short). I truncated some of the irrelevant graphs from this chart, but the point is clear, they too (citing Alvheim) found that LA is obesogenic.
Alvheim’s finding also elucidates some previous studies.
In 1993, (Pan & Storlien, 1993) found that different dietary lipids induced different amounts of obesity:
“Despite isocaloric feeding, weight gain was lower (P < 0.001) in rats fed the more highly saturated ET-L [Edible Tallow (from beef)] diet (69 +/- 8 g) than in those fed either the high (n-9) fatty acid OL-L [Olive oil] diet (93 +/- 2 g) or the high (n-6) fatty acid SAF-L [Safflower oil] diet (108 +/- 4 g).”
In 2004 (Ghosh et al., 2004) (a different Ghosh, btw) published a paper looking at what happened when you added n-6 PUFA to a standard rat chow diet.
It turns out what happens is obesity. I covered this paper at length (Goodrich, 2018) so I won’t do more here than include a graph I made for that post. P in PC and PD (the right two columns) stands for n-6 PUFA, it robustly made the mice fat, along with several other bad outcomes.
But Wait, You Mentioned a Mechanism?
(DiPatrizio et al., 2011) looked at what causes a food to be palatable, in the Food Reward Hypothesis sense of the word.
“The proposed role of endocannabinoids in the hedonic evaluation (‘liking’) of palatable foods (7, 8) prompted us to ask whether these lipid mediators might be involved in the positive feedback mechanism…”
They investigated endocannabinoid signaling in the gut of rats fed various foods, through a procedure known as “sham feeding”, where food consumed by the rats is drained from the stomach prior to it reaching the small intestine; hence the rats weren’t allowed to digest the food.
“Surprisingly, fat sham feeding [of corn oil] selectively increased levels of 2-AG and anandamide in the jejunal segment of the gastrointestinal tract (Fig. 1A and B, 1)... A similar response was elicited by a nutritionally complete liquid diet (Fig. 1 A and B, 2), whereas sugar (Fig. 1 A and B, 3) or protein solutions were ineffective (Fig. 1 A and B, 4).”
Given the list of ingredients in Ensure—"Corn Maltodextrin, Sugar, Milk Protein Concentrate, Blend of Vegetable Oils (Canola, Corn)—the intermediate performance of Ensure is not surprising (Abbott Laboratories, Inc., 2020).
I’m not sure when Ensure was introduced to the market, but its use as a “weight-gaining” solution for the elderly was anticipated in this 2004 paper. After noting that, “At each age the PUFA [sunflower oil] diet was associated with greater weight gain than the SFA [beef tallow] diet,” the authors noted:
“However, the short-term feeding of a PUFA diet may provide a therapeutic option to mitigate weight loss associated with aging.” (Woudstra et al., 2004)
Now you’ll note these papers I cite keep mentioning 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide or AEA). As indicated by the bolded part of those two names, both these chemicals are derived from arachidonic acid (AA), an n-6 fat produced in the body from LA. These two chemicals are derived from AA-containing phospholipids in the body, and are crucial to the body’s reward mechanisms, including such processes as addiction (Wang et al., 2015).
“For example, small-intestinal levels of 2-AG and anandamide rise during food deprivation and fall upon refeeding, suggesting that they may signal energy imbalance and promote caloric intake (21, 23).” (DiPatrizio et al., 2011)
Both are involved in the control of feeding:
“We have also shown what we believe to be the first evidence of behavioural activity of 2-AG: specifically we demonstrated that 2-AG can reliably stimulate eating.” (Kirkham et al., 2002)
And:
“When 2-AG was injected into the nucleus accumbens shell, a limbic forebrain area strongly linked to eating motivation, this potently, and dose-dependently, stimulated feeding.” (Witkamp, 2018)
And:
“Overall, the present experiments indicate that administration of both exogenous [THC from marijuana] and endogenous cannabinoids [AEA] selectively induces overeating by stimulating those systems which normally control feeding.” (Williams & Kirkham, 2002)
2-AG and AEA are part of the endocannabinoid system (ECS), so named because it was initially discovered when exploring the effects of cannabinoid plants like marijuana on the body.
“The endocannabinoid system, which comprises endogenous signaling molecules (endocannabinoids) and receptors responsible for their actions [cannabinoid 1 and 2 receptors (CB1Rs and CB2Rs)] and enzyme systems that catalyze their biosynthesis and degradation, is a key regulator of palatable food intake (for review, see refs. 8, 9), and pharmacological activation of this system increases the consumption of fatty foods in rats (10).” (DiPatrizio et al., 2013)
DiPatrizio et al. in their 2011 paper refer to “fat” generically, but after Alvheim et al. 2012, they did more work in which they narrowed down the type of fat.
Alvheim had demonstrated that increasing LA in the absence of n-3 fats induced obesity, and measured 2-AG and AEA, but did not do an intervention that would demonstrate that mechanism was causal. DiPatrizio did, in fact they did several interventions.
DiPatrizio had already demonstrated in 2011 that fat stimulated the EC, and that this effect was mediated by the brain—as the effect was dependent on an intact nervous system between the gut and the brain. They additionally demonstrated that an antagonist of the EC, rimonabant, blocked this effect, as did URB 447, which is a peripherally-active antagonist. (An antagonist works against, an agonist promotes.)
In the 2013 paper DiPatrizio looked at several different fats, not only the PUFA-rich fats they had used in the previous paper. “These included stearic acid [18:0 fatty acid (FA)], oleic acid (18:1 FA [OA]), linoleic acid (18:2 FA [LA]), and alpha-linolenic acid (18:3 FA [ALA]).” (They use slightly non-standard terminology, referring to OA as a monoenoic fat, to LA as dienoic, and to ALA as a polyunsaturated fat. All this means is that OA has a single unsaturated bond (monounsaturated) and linoleic has 2 unsaturated bonds, and ALA has more than two. Typically anything with more than one unsaturated bond is referred to as polyunsaturated.)
(A. R. Alvheim et al., 2012) reduced both MUFA and n-6 PUFA from what was found in the standard obesogenic diet. (DiPatrizio et al., 2013) found that both MUFA OA and n-6 PUFA in LA stimulated the ECS:
“In the present investigation, we report that orosensory exposure to the free fatty acid component of dietary fat, rather than fat texture, mobilizes endocannabinoid substances in the rat jejunum; this effect is mediated by monoenoic (18:1 FA) [OA] and dienoic (18:2 FA) [LA] fatty acids, but not saturated (18:0 FA) or polyunsaturated (18:3 FA) fatty acids; rats display, when given a choice, a strong preference for 18:2 FA vs. a nonnutritive MO that contains no fatty acids, but has similar textural properties to that of nutritive oils; and this preference for unsaturated fatty acids is impaired by pharmacological blockade of peripheral CB1Rs” (DiPatrizio et al., 2013).
Amusingly, stearic acid (18:0), one of the saturated fats long blamed for causing obesity, had no effect on appetite. In fact, the animals preferred indigestible mineral oil (MO) alone to MO + stearic acid!
“We next evaluated whether rats prefer 18:0 FA, the intake of which does not modify endocannabinoid levels in jejunum (Figs. 2A and 3A), over MO in the 2-bottle choice sham-feeding test. In contrast to 18:2 FA, rats preferred emulsions containing only MO to those containing both MO and 18:0 FA (Fig 4C: vehicle, MO vs. 18:0 FA, P<0.05, n=5).”
Having found that LA seemed to best stimulate the EC, they continued:
“...to assess the preference for 18:2 FA, the most abundant fatty acid constituent of corn oil (28) and the fatty acid that most robustly causes accumulation of jejunal endocannabinoids (Figs. 2C and 3C), vs. MO, and the role of gut endocannabinoid signaling in determining the preference for dietary fat.”
And they found that blocking the ECS through either of two different ECS antagonists reduced the preference for OA and LA.
“In the present investigation, we report that orosensory exposure to the free fatty acid component of dietary fat, rather than fat texture, mobilizes endocannabinoid substances in the rat jejunum; this effect is mediated by monoenoic (18:1 FA [OA]) and dienoic (18:2 FA [LA]) fatty acids, but not saturated (18:0 FA [stearic]) or polyunsaturated (18:3 FA [ALA]) fatty acids; rats display, when given a choice, a strong preference for [LA] vs. a nonnutritive MO that contains no fatty acids, but has similar textural properties to that of nutritive oils; and this preference for unsaturated fatty acids is impaired by pharmacological blockade of peripheral CB1Rs.”
This paper demonstrates that the fat that apparently most significantly affects the preference for specific foods, via the ECS is linoleic acid, and that other fats have a smaller or no effect. We will discuss OA and eating and satiety further below.
At the same time Alvheim released their paper, another group was working on a similar problem. They tested the effect of rimonabant and another, novel ECS blocker, NESS038C6 (Mastinu et al., 2012). Mastinu raised four groups of mice on the cookie dough diet, D12492. They then treated three of the four groups: one was put on a chow diet (green marking in graph), and the other two were put on rimonabant (aka SR141716) or NESS038C6 (orange markings). Comparing the group indicated in red, one can clearly see that the drugs are not as effective as the chow diet, but are close. (One can also see that the chow-fed mice ate more, yet weighted less, with lower fat mass and BMI.)
So this confirms the effects on ECS that Alvheim (A. R. Alvheim et al., 2012) saw, and, further, confirms their supposition:
“We postulated that in lieu of a better antiobesity drug, addressing an underlying cause of endocannabinoid hyperactivity may prove to be a viable and safe preventative alternative for decreasing obesity.”
Further, it confirms the mechanism proposed for the confounding effect of dietary n-3:
“Decreasing AA-PL in liver and erythrocyte by adding 1 en% from EPA and DHA reduced 2-AG + 1-AG and AEA levels, reduced feed efficiency and reversed the adipogenic effects of the 8 en% LA diets.”
It also shows that seed oils (LA) may be a fundamental cause underlying the modern obesity epidemic.
What is missing is an explanation for what causes dysregulation of the food reward system:
This is, as indicated, a fascinating paper. (Han et al., 2018) They confirm many of the findings from the papers cited above, notably (DiPatrizio et al., 2011, 2015), who also found that the vagus nerve must be intact for the intestinal food-reward circuit to work (although see the RYGB section for an update):
“Notably, severing the vagus nerve below the diaphragm abolished the ability of fat to initiate small-intestinal endocannabinoid production (Fig. S2), which indicates that neural communication from the brain to the gut via the vagus nerve is necessary to drive this biochemical reaction. These findings suggest that cephalic signals elicited by sham feeding of fat, but not other nutrients, selectively mobilize endocannabinoids in the upper gut through a mechanism that is mediated by efferent vagal fibers.” (DiPatrizio et al., 2011)
Of course, as we’ve seen, it wasn’t “fat”, it was LA or OA.
But there was a notable difference. DiPatrizio used corn oil (Baur & Brown, 1945) to stimulate the EC, and Han used Intralipid (Sigma-Aldrich, Inc., 2020), which is based on soybean oil. So what’s the difference between the two seed oils?
Virtually nothing.
So even a seminal paper demonstrating food reward and the brain/gut connection relies on LA-rich seed oils to demonstrate their mechanism.
(If you are wondering about that difference in linolenic acid (ALA) between the two diets, see (Enos et al., 2014).)
A final—and incredibly important—note on this line of research is provided by a paper looking at a model of obesity using a genetically-modified mouse. I’ve mentioned CB1R above via quotations, so a brief explanation: The CB1R is a CannaBinoid 1 Receptor, the feature of a cell that allows it to receive the signal delivered by cannabinoid chemicals such as THC, 2-AG, and AEA. Rimonabant is a CB1R antagonist, in that it impairs the ability of the CB1R to receive these signals. (There is a CB2 receptor also, but it doesn’t seem to be as central to this process.) (Ravinet Trillou et al., 2004) used a CB1R-knock-out mouse model, it had the gene required to enable the CB1R removed. This was a follow-up to their 2003 paper (Ravinet Trillou et al., 2003), “[Rimonabant] had no effect in CB1 receptor knockout mice, which confirmed the implication of CB1 receptors in the activity of the compound.”
“When given a high-energy regimen, the adiposity of CB1-/- [knock-out] mice remains low in contrast to that observed in the wild-type animals. Although fat deposition slightly increases with the diet, it still remains lower than that of control animals fed the standard diet alone. These data suggest that inactivation of CB1 receptors is capable of counterbalancing the development of the obesity induced by a high-energy regimen.”
So this suggests that there is some obesogenic element that remains in the HFD, although it is small. Ravinet Trillou et al use a different high-fat diet, Envigo TD.97366, which has an intermediate level of LA (D. Martin, personal communication, 2015) compared to the two high-fat diets used in (A. R. Alvheim et al., 2012).
What’s also fascinating, is that the CB1 knockout mouse still preferred the HFD, although the effect was somewhat delayed.
“When analyzing the composition of the meal, the low level of fat storage in CB1-/- mice does not seem to be related to a difference in the nature of the diet ingested. During the first day of the free-choice paradigm, CB1-/- mice ate about two-thirds of their daily energy from the HFD, while the wild-type mice chose only the high-fat food. However, after the second day of exposure to the two diets, mutant mice consumed exclusively the HFD like their wild-type litter-mates as demonstrated by the data collected during the first 4 weeks on study and after 11 weeks on regimen (data not shown). Thus, the free-choice regimen finally results in a high-fat feeding in the two strains. Therefore, inactivation of CB1 receptors does not prevent mice from choosing the highly palatable diet, but prevents excessive eating.” (Ravinet Trillou et al., 2004)
Emphasis mine. While this is further confirmation of the Food-Reward Hypothesis, it certainly casts it in a new light. The highly-palatable HFD depends on the ECS to induce obesity through over-consumption, but not to prefer that diet.
We learn two things from this study: 1) the HFD is more obesogenic than the STD diet regardless of CB1 status, and 2) the CB1 knock-out mice had lower fat mass on both the obesogenic and control diets (see Figure 1 from (Ravinet Trillou et al., 2004), indicating that the HFD used is fundamentally obesogenic, there’s a mechanism operating in addition to the ECS, and that ECS is a fundamental regulator of fat mass, even in the absence of an obesogenic diet.
We’ll look further into what might make the HFD fundamentally obesogenic later, in the section on HNE.
Looking specifically at the liver, a high-fat diet increases the endocannabinoid anandamide, lipogenesis, obesity, and decreases energy expenditure, but not in mice that are deficient in CB1 or treated with rimonabant. CB1 knockout mice also have a lower adiposity and fatty liver than wild-type mice. (Osei-Hyiaman et al., 2005). 2-AG may be specifically harmful to the liver, regardless of the CB1 receptor pathway, and despite an anti-inflammatory effect generally (Siegmund et al., 2007). These effects may be mediated by the direct negative effects cannabinoids have on mitochondria (Athanasiou et al., 2007; Walker et al., 2021). As mitochondrial damage and dysfunction appears to be fundamental in obesity, this may be an important connection (Rogge, 2009).
This indication was pursued by the same group in (Jbilo et al., 2005), and they found that indeed, the ECS regulates a wide variety of gene expression and metabolic pathways in the context of fat metabolism, not solely obesity.
“Importantly, the gene modulations obtained in CB1 receptor knockout mice fed a high-fat diet were similar to those observed in obese mice treated with the CB1 antagonist SR141716 [rimonabant]. This supports our hypothesis that the SR141716-dependent gene modulations resulted from a CB1 receptor-mediated process….
“The coordinated induction of all of these genes acting at different levels of the fatty acid catabolism pathway strongly suggested that treatment with [rimonabant] favored lipolysis and thereby reduced fat storage in WAT. Activation of a redox pathway is often associated with the induction of β-oxidation and oxidative phosphorylation, as this leads to increased reactive oxygen species (ROS) production. Consistent with this observation, two antioxidant enzymes were induced by [rimonabant], that is, glutathione peroxidase 3, (GPX3) and flavin-containing monooxygenase (FMO1).”
Summing up this line of experiments, we learn that “Cannabinoid CB1 Receptors in the Intestinal Epithelium Are Required for Acute Western-Diet Preferences in Mice” (Avalos et al., 2020), which uses both gene knockouts and pharmacological antagonism to demonstrate that animals without an active ECS in the gut are not susceptible to diet-induced obesity.
“In summary, these studies extend our understanding beyond central roles for the endocannabinoid system in intake and reward value of palatable food [68–84], and provide evidence that CB1Rs in the intestinal epithelium are an integral component of a gut–brain axis that controls dietary preferences.” (Avalos et al., 2020)
Thus the dysregulation induced by LA-induced stimulation of the ECS extends beyond over-eating to an alteration of fat-storing and oxidation systemically. The increase in glutathione will be discussed in the section on HNE, below.
Is Olive Oil Bad for You?
The effect of oleic acid (OA) stimulating the ECS shown above is surprising. While there are papers that hint that excess OA (the primary fat in olive oil) can be harmful:
“Mice were fed the [Western Diet (WD)] supplemented with either olive oil (OO), EPA, DHA, or EPA + DHA for 16 wk. WD + OO feeding induced a severe NASH phenotype, characterized by robust hepatosteatosis, inflammation, oxidative stress, and fibrosis.” (Depner et al., 2013)
In general OA seems to be protective; for instance a genetically-modified soybean oil containing higher amounts of OA: “GM soybean oil causes less obesity and insulin resistance but is harmful to liver function…” (Pittalwala, 2017)
OA—but not other fats—metabolizes in the small intestine to oleoylethanolamide (OAE) (DiPatrizio et al., 2011; Guijarro et al., 2010), which has the effect of suppressing appetite, in both animals (Fu et al., 2005; Naughton et al., 2013; Schwartz et al., 2008; Tellez et al., 2013), and humans (Grosshans et al., 2014; Laleh et al., 2018; Mennella et al., 2015)
(Tellez et al., 2013) is uniquely interesting, as they examined OAE in the context of D12492, making the results easily comparable to our other studies. (Tellez was later a co-author on the “Neural Circuit” paper (Han et al., 2018))
“We specifically hypothesized that reduced synthesis of the diet-derived satiety messenger oleoylethanolamine [OEA (17,19)] links excessive lipid intake to dopamine deficiency. …we confirmed that a high-fat diet produces in mice significant reductions in intestinal OEA synthesis, whereas no changes are detected in other tissues (fig. S1). Consistently, sub chronic intraduodenal OEA treatment was sufficient to decrease weight gain and fat intake in high-fat–fed mice (fig. S2).” (Tellez et al., 2013)
This is a surprising result, as the D12492 diet has about 22% energy as OA (Liu, 2013), while the control diet used, D12450B, has 10% energy as total fat. (Research Diets, Inc., 2006a) Clearly OA quantity alone is not what is driving OAE production. Nevertheless, infusion of OEA led to a dramatic decline in body weight, and ceasing infusion led to a rapid regression to the obese state.
Happily, the idea was pursued.
“These findings indicate that the conversion of dietary oleic acid into OEA is selectively disrupted in diet-induced obesity [via D12492].” (Igarashi et al., 2015)
Igarashi also found that a high-sugar diet was not good for similar reasons, as this was unfortunately their control, but:
“Interestingly, 7-day feeding of high fat-high oleic acid diet (45 kcal%, the diet contained oleic acid rich-olive oil) did not reduce intestinal OEA levels, while reducing levels of PEA and LEA [30]. We interpret these findings to indicate that under short-term feeding conditions both fatty acid composition and quantity of dietary fat are critical regulators of OEA-dependent signaling.”
Igarashi did not, alas, discover a mechanism for why this happens, although they do have some interesting ideas. “More study required…”
However, they did establish that it’s the ingredients in D12492 that are at issue, not olive oil, per se. While olive oil may stimulate the feeding signals, it also can signal satiation. As OA is a common fat in animal fats, this would be a useful adaptation, as fat is our most important source of energy in our natural environment.
D12492 thus stimulates the ECS via 2-AG, inducing overeating and obesity, while impairing the ability to produce the satiation signal OAE. This seems to have some other bad consequences, but those are outside the scope of this discussion. (Tutunchi et al., 2019)
Another point about OEA to follow…
But This Is in Mice, Not Humans, Right?
Typically, when one is discussing a mechanism such as this, one looks at lots of animal data and tries to extrapolate to humans, which is extremely problematic, as most such extrapolations fail.
In this case, however, we are determining the mechanism behind a human-approved anti-obesity drug, rimonabant.
“Large randomized trials with rimonabant have demonstrated efficacy in treatment of overweight and obese individuals with weight loss significantly greater than a reduced calorie diet alone. In addition, multiple other cardiometabolic parameters were improved in the treatment groups including increased levels of [HDL], reduced triglycerides, reduced waist circumference, improved insulin sensitivity, decreased insulin levels, and in diabetic patients improvement in [HbA1c].” (Bronander & Bloch, 2007)
It was a miracle drug, in other words. Sadly, it also inspired people to want to kill themselves, and so was withdrawn from the market. Apparently rodents in labs have limited opportunities for suicide, and so this side effect was missed, although since (Kirkham & Williams, 2001) noted the effects on mood of endocannabinoids years earlier, “Interestingly, improvements in mood preceded the changes in appetite and nausea ratings, again indicating the need for more thorough assessment of the wider psychological and behavioural effects of these treatments.” A bit of caution ought to have been exercised.
Most of the other ECS blockers mentioned above are “peripheral” ECS blockers, meaning they block ECS activation in the body (or ideally just the gut) but not the brain, but they are mediating the same mechanism as rimonabant, blocking the uptake of endocannabinoids like 2-AG and AEA. While the failure of rimonabant killed the ECS blocker business, research continues to find something as effective without the nasty side effect.
The effectiveness of this mechanism in stimulating reward, and the usefulness of rimonabant in controlling it, is well demonstrated by this study in primates:
“Intravenous 2-AG was a very effective reinforcer of drug-taking behavior, maintaining higher numbers of self-administered injections per session and higher rates of responding than vehicle across a wide range of doses. To assess involvement of CB1 receptors in the reinforcing effects of 2-AG, we pretreated monkeys with the cannabinoid CB1 receptor inverse agonist/antagonist rimonabant... Rimonabant produced persistent blockade of 2-AG self-administration without affecting responding maintained by food under similar conditions. Thus, 2-AG was actively self-administered by monkeys with or without a history of cannabinoid self-administration, and the reinforcing effects of 2-AG were mediated by CB1 receptors.” (Justinová et al., 2011)
While the sorts of studies done in lab animals obviously cannot be done in humans, we have the demonstrated efficacy of rimonabant to suggest that it is affecting the same pathway in humans.
This effect of AA may be related to the lack of DHA in high-n-6 oils (A. R. Alvheim et al., 2012; Garg et al., 1992). This may also explain the difference in obesity between China and Japan, as the Japanese consume more fish, rich in DHA (Rossmeisl et al., 2012; Saito et al., 2011).
Moreover, a mutation in the ability to break down endocannabinoids (in the fatty acid amide hydrolase, FAAH, enzyme) strongly correlates with obesity in humans (Sipe et al., 2005, 2010). Pharmacological inhibition of this enzyme in the brain stimulates eating acutely, and blockage of the CB1 receptor pharmacologically (with an analogue of rimonabant) blocks this effect, in rodents (Soria-Gómez et al., 2007). Typically endocannabinoids are broken back down to arachidonic acid and re-stored. The inability to do so suggests a chronic overstimulation of the ECS.
(Garg et al., 1992) demonstrated that the effects of LA consumption mimic the effects of food deprivation. Either reducing food intake or increasing intake of LA increased the amount of Δ6 desaturase, the enzyme responsible for converting LA to AA, and thus increasing the pool of substrate available for creating the hyperphagia-inducing endocannabinoids. They looked at the small intestine (specifically the jejunum), as did (DiPatrizio et al., 2013), while (A. R. Alvheim et al., 2012) looked only at the liver and the brain, however they concisely summarized Garg’s findings (despite not referencing Garg):
“These results indicate that reducing the endocannabinoid precursor pool of AA-PL by either lowering dietary LA or raising EPA/DHA is similarly effective in lowering 2-AG + 1-AG and AEA concentrations.” (A. R. Alvheim et al., 2012)
2-AG and AEA are hunger hormones: they go up when you have not eaten, and down when you are fed (DiPatrizio et al., 2015), and OEA is the reverse (Petersen et al., 2006).
Like THC, which is used as a drug (dronabinol) to stimulate eating in humans (Beal et al., 1997; U.S. National Library of Medicine, 2017), AEA and 2-AG stimulate eating in animal models. They are found in high levels in obese humans as seen in (Côté et al., 2007; Gatta-Cherifi et al., 2012; Little et al., 2018). (Levels of the two chemicals vary depending on the compartment measured, see chart from (Matias et al., 2008).)
(Gatta-Cherifi et al., 2012) conducted a feeding study:
“Both normal-weight and obese subjects had a significant preprandial AEA peak. Postprandially, AEA levels significantly decreased in normal-weight, whereas no significant changes were observed in obese subjects. Similarly, PYY levels significantly increased in normal-weight subjects only… Postprandial AEA and PYY changes inversely correlated with waist circumference, and independently explained 20.7 and 21.3% of waist variance.” (Gatta-Cherifi et al., 2012)
(Garg et al., 1992) demonstrates that this effect upon the ECS influenced by diet is dependent, to some extent, upon the presence of both AA and LA substrates in the gut, as confirmed in (Petersen et al., 2006), which is sufficient to control the hyperphagia response to LA (DiPatrizio et al., 2011)
The effect of a high-n-6 diet to impair production of the satiety signal OEA (Igarashi et al., 2015) may alone account for the induction of obesity demonstrated by this line of research, as clearly the package of inducing a feeding signal and reducing a satiety signal has sufficient explanatory power (Mennella et al., 2015) It should thus be no surprise that OEA is being explored as an obesity treatment (Brown et al., 2017; Laleh et al., 2019; Payahoo et al., 2019; Romano et al., 2014; Tutunchi et al., 2020).
However, the avenues open to researchers are limited in humans, especially now that rimonabant has been withdrawn from the market (this scotched an interesting study on the effects of dronabinol, (Gorelick et al., 2011; Huestis et al., 2007), a synthetic THC used to induce eating in humans, for instance (Beal et al., 1997; U.S. National Library of Medicine, 2017). You also can’t puree a person’s intestine to determine the fatty acid composition of the phospholipids (Garg et al., 1992).
(Joosten et al., 2010) looked at fat-derived endocannabinoids: “Plasma anandamide and other N-acylethanolamines are correlated with their corresponding free fatty acid levels under both fasting and non-fasting conditions in women”:
“Fasting AEA levels correlated positively with total FFA… and with arachidonic acid levels … Furthermore, AEA… and OEA levels… were also positively related with BMI.”
Similarly:
“Plasma 2-AG, but not AEA, levels correlated positively with BMI, waist girth, [intra-abdominal adiposity (IAA)] measured by computed tomography, and fasting plasma triglyceride and insulin levels... Obese men with similar BMI values (X30 kg/m2) but who markedly differed in their amount of IAA… exhibited higher 2-AG levels in the presence of high IAA. No difference in 2-AG concentrations was observed between obese men with low levels of IAA vs nonobese controls.” (Côté et al., 2007)
AA, the direct precursor to 2-AG, is also related to obesity (Karlsson et al., 2006; Saito et al., 2011; Tsurutani et al., 2018) but not in (Blüher et al., 2006), which nonetheless found:
“Circulating 2-arachidonoyl glycerol (2-AG) was significantly correlated with body fat..., visceral fat mass …, and fasting plasma insulin concentrations… Our findings suggest that abdominal fat accumulation is a critical correlate of the dysregulation of the peripheral endocannabinoid system in human obesity.” (Blüher et al., 2006)
Observing that:
“After adjusting for age, physical activity, BMI, waist circumference, and fat mass, higher levels of AEA and 2-AG were observed in participants who were in the highest quintile of the Western pattern (P < 0.05). Also, in both unadjusted and adjusted models, significantly lower levels of AEA and 2-AG were detected in the women of the highest quintile of the healthy pattern (P < 0.01). Moreover, there was no significant association between "traditional" pattern and AEA and 2- AG levels in both unadjusted and adjusted models (P > 0.05).” (Yagin et al., 2020)
These authors note the increase in seed oils in the modern diet:
“The addition of vegetable oils that contain a relatively high amount of ω−6 fatty acids contributes to an excess ratio of ω − 6 to ω − 3. Surplus intakes omega-6 vegetable oils are associated with reduction of EPA/DHA incorporation into cellular membranes, increasing the AEA and 2-AG production [46, 47].” (Yagin et al., 2020)
Of course correlation is the roughest form of science. We’re interested in causation. There are a number of studies that look at what happens when you alter the fats consumed by people.
(Flint et al., 2003) looked at fat type and appetite in a short-term feeding study, finding no difference acutely in overweight individuals, but not looking at any hormonal impacts, or the preexisting hormonal status of the individuals. Appetite and energy balance were the factors observed, but they were not directly looking at obesity, and they observe this study was an outlier:
“The lack of differences among high-fat test meals predominantly constituted of MUFA, PUFA, or TRANS is in opposition to other studies undertaken to investigate the effect of different dietary fats on appetite or energy metabolism (17–21).”
While not explicitly looking at obesity, (Hodge et al., 2007), in a study looking at the effects of seed oils on diabetes, noted that obesity (via BMI and waist-hip ratio) was correlated with LA intake, although inversely correlated with blood (phospholipid) content of LA. (Cases were those with diabetes that appeared during the study (incident), this was the group with the higher intake of seed oils.)
In a contrary finding to (Flint et al., 2003), (Mennella et al., 2015) looked at the effect of three different fats: sunflower oil, high-oleic sunflower oil, and virgin olive oil.
The title of the paper describes their findings concisely: “[OA] content of a meal promotes [OEA] response and reduces subsequent energy intake in humans.” However, they also found:
“Interestingly, increased fullness and satiety compared to baseline were found between 30 min and 60 min after meals containing HOSO and VOO, respectively, but not after SO. These perceptions were prolonged at 120 min only following VOO consumption.”
VOO had the lowest LA concentration, suggesting an interplay between the relative amounts of the two fats. This confirms the observation above that OEA seems to play a crucial role in appetite regulation, while still suggesting an independent role of LA.
(Kaviani & Cooper, 2017) published a “comprehensive review” of, “Appetite responses to high-fat meals or diets of varying fatty acid composition” in the context of obesity. However, they don’t mention any of the research concerning rimonabant, the ECS, AEA, OEA, or 2-AG. This is rather inexplicable, given that rimonabant had been a human-approved anti-obesity drug, affecting the appetite pathway! As (Bronander & Bloch, 2007) put it, “The ECS is intimately involved in appetite regulation and energy homeostasis, which makes it an intriguing target for pharmacological treatment of obesity…” Welcome to the world of nutrition research, where much contrary evidence is often simply ignored. Having removed that body of evidence from consideration, (Kaviani & Cooper, 2017) found, “Based on those studies, the majority (6 out of 11) indicate that PUFAs, followed by MUFAs, induce the greatest satiety…”
(Naughton et al., 2018) thus looked into the question. Having just authored a paper titled “Linoleic Acid and the Pathogenesis of Obesity” (Naughton et al., 2016), which specifically discussed the role of the ECS and appetite, which was of course not mentioned in (Kaviani & Cooper, 2017), they must have been quite astonished, and replied with a paper:
“Additionally, the role of these specific fatty acids (FA) in acutely modulating satiety-regulating hormones and perception of hunger and prospective food intake is yet to be fully elucidated, with no clear associations identified between them and appetite-modulating hormones [28] [reference to (Kaviani & Cooper, 2017)]. Therefore, this pilot study aimed to assess the acute (2 h) response of appetite-regulating hormones, metabolic markers, and cytokines, as well as self-reported appetite parameters, following the consumption of mixed composition meals containing high levels of two of the most common dietary fats (LA or OA), in comparison to an energy-matched predominately carbohydrate-containing meal, in overweight or obese men and women.”
And while only a pilot study, and thus lacking in some respects, they found:
“In this study, using carefully designed meals with matched energy from macronutrients and matched contents of the specifically elevated fatty acids in the two high-fat meals, we were able to show regulation of postprandial appetite markers and perceived appetite by either OA or LA.”
Furthermore:
“This possible indication of the promotion of hedonic food intake can be stimulated by ghrelin, which was increased following consumption of the LA meal in the current study. This is an important factor when considering modern environments of abundant, high-fat, and especially high-LA, palatable foods [67,68].”
Of course short-term appetite studies can only inform so far about n-6 involvement in obesity. There’s no indication that a single shot of seed oils will make you obese, any more than a single cigarette will give you lung cancer. Unfortunately, given the long involvement of industrial seed oils in the human diet (Blasbalg et al., 2011), there are very few populations that are naïve to them. However, there are a number of pilot studies looking at the reduction of seed oils and the effect on obesity and related conditions.
Non-Alcoholic Fatty Liver Disease (NAFLD) is a now-common liver condition that is highly correlated with obesity and Type 2 Diabetes Mellitus (T2DM) (Lindenmeyer & McCullough, 2018). NAFLD is thought to have a close relation to seed oil intake—"Markers of lipid oxidation are of particular interest given their principal role in pathogenesis of [severe NAFLD]” (Tincopa, 2020), so (Nigam et al., 2014) looked at how different fats would affect NAFLD and related markers.
“Post-intervention weight and BMI (ANOVA, P = 0.01) of the olive oil group were significantly decreased from values for the control oil group…” The Canola group, while not reaching “statistical” significance, indicate a middle ground in dose response between the high and low n-6 arms. Additionally, “In the current study we showed that intervention with olive and canola oils as a cooking medium was effective in reducing the liver span and grade of fatty infiltration in addition to improving insulin sensitivity.” Again, we see it’s hard to detect a signal from the saturated fats in the oils, or, for that matter, from the 55-70% of carbohydrate in the diets.
In parallel, (Maciejewska et al., 2015, 2018) undertook a similar pilot study:
“The aim of this study was to compare the profile of eicosanoids (AA and LA derivatives) and biochemical parameters in patients with NAFLD… A six-month dietary intervention was undertaken to achieve a reduction in fatty liver.”
Since these eicosanoids are known to be specifically derived from n-6 fats, the intake of these fats was specifically reduced, although the papers do not describe by how much. PUFA intake was described as, “The total omega-3 and omega-6 fatty acids consumption was approximately 0.5% E for omega-3 and 4% E for omega-6.” The diets were individually tailored, however, and the ranges of fat and carbohydrate intake, and the fact that some diets may have been calorie restricted makes the results somewhat difficult to interpret. However, they did measure markers of seed oil intake, specifically “13-hydroxyoctadecadienoic acid (13-HODE, 0.76±0.23 before and 0.42±0.08 after the diet, <0.001), 9-hydroxyoctadecadienoic acid (9-HODE, 0.52±0.25 before and 0.28±0.07 after the diet, P<0.001)…” which have been shown by other authors to be dependent on dietary intake of seed oils (Mann et al., 2018; Ramsden et al., 2012; Strassburg et al., 2014). This indicates that this was indeed a reduction of seed oil study.
“Following the six-month dietary intervention, hepatic steatosis resolved completely in all patients. This resulted in a significant decrease in the concentrations of all eicosanoids (LX4, 16-HETE, 13-HODE, 9-HODE, 15-HETE, 12-HETE, 5-oxoETE, 5-HETE) and key biochemical parameters (BMI, insulin, HOMA-IR, liver enzymes).”
Concentrations of the key biochemical parameters also significantly changed, including body mass index (BMI, decreased from 30.7±8 to 28.4±6.6 after the diet, P<0.05)…
The reduction of BMI and those parameters highly correlated with obesity are, in my view, indicative of an underlying process that is affecting all the parameters.
A further interesting study was undertaken by (Van Name et al., 2019, 2020). Sadly:
“We meticulously controlled for weight to avoid the confounding factor of weight loss as a mechanism to reduce intrahepatic fat and, indeed, the mean BMI of the cohort did not change significantly from week 0 to week 12 of the intervention.”
Sigh. More sadly, they didn’t even track the calories that had to be altered to maintain BMI, which would have added a fascinating dimension to the results, without affecting the weight-stability goal. That said, the data shows a slight reduction in weight, which could of course be noise, and a slight reduction in both subcutaneous and visceral fat.
“This study has shown, to our knowledge for the first time, that a nonpharmacologic, food-based dietary intervention high in n–3 and low in n–6 PUFA intake improves fatty liver disease in obese adolescents, and restores liver fat content to normal in one-third of participants, in the absence of weight loss. The observed improvement in lipids, lipoprotein concentrations, and insulin sensitivity at the end of the study demonstrates a beneficial impact of a low n–6:n–3 PUFA ratio diet on both diabetes and cardiovascular disease risk factors.”
In this case the non-fat energy portion of the diet was “50%–55% daily total calories from carbohydrate”. One wonders what would have happened if the kids had also lost weight.
(Garaulet et al., 2001) had noted that “Central obesity was positively associated with n-6 polyunsaturated fatty acids and inversely associated with monounsaturated fatty acids and n-3 polyunsaturated fatty acids in adipose tissue (P < 0.05),” so this finding shouldn’t be entirely surprising
In 2019 Kevin Hall, a researcher at the National Institutes of Health, did a fascinating study looking at the effect of ultra-processed foods on obesity (Hall et al., 2019). The definition of “ultra-processed food” used was the NOVA system (Monteiro et al., 2018), and while there are many issues with that classification system (some of which are described in Hall’s paper), they are outside the scope of this discussion.
Hall attempted to control dietary variables between the two arms, but this was not completely possible.
“While we attempted to match several nutritional parameters between the diets, the ultra-processed versus unprocessed meals differed substantially in the proportion of added to total sugar (~54% versus 1%, respectively), insoluble to total fiber (~16% versus 77%, respectively), saturated to total fat (~34% versus 19%), and the ratio of omega-6 to omega-3 fatty acids (~11:1 versus 5:1)”
What they found was a clear increase in fattiness on the ultra-processed diet (PD), and a decrease in the unprocessed one (UD). The PD had an n-6 intake of 7.29 E%, and n-3 of 0.54 E%. UD had 10.35 E% and 1.98 E%, respectively.
Hall also noticed a hormonal difference between the two diets:
“Interestingly, the appetite-suppressing hormone PYY increased during the [UD] as compared with both the [UPD] and baseline. Also, the hunger hormone ghrelin was decreased during the [UD] compared to baseline.”
These results are comparable to the results found in (A. R. Alvheim et al., 2012), where a higher n-3 intake (of 1 E%, vs ~2 E% in this paper) was found to be protective against obesity in a rodent model, and (Naughton et al., 2018), where a higher n-6 intake was found to affect the hunger hormone ghrelin in humans. Unfortunately, these three studies are not entirely comparable, as they measured different aspects, so a 1-to-1 comparison is not possible. They are consistent, however, so far as they go.
Hall, in his apparent quest to touch all the dietary third rails, did another paper with a bearing on this question (Hall et al., 2021). This paper looked at an Animal-Based, Low-Carb diet (LC) vs. a Plant-Based, Low-Fat diet (LF). Here they found a much greater reduction in fattiness on the LF diet — which was a surprise to many, myself included.
Calories consumed are not directly reported in this paper, but Dr. Hall was kind enough to provide them (K. D. Hall, personal communication, May 6, 2021). LC dieters consumed ~17.55 E% n-6, and ~1.62 E% n-3, and LF dieters consumed ~3.24 E% and ~0.45 E%, respectively. Sadly, the paper doesn’t report any appetite-related hormonal figures. Lacking for data, these results are consistent with the hypothesis, as both the low n-6 and high n-3 wings lost weight, with the low n-6 wing losing more, but it’s tough to draw a further conclusion than that. (The high intake of n-6, predominantly a plant-based fat, in the animal-based wing was apparently from mayonnaise, which is typically made from soybean oil. That’s a bit of a confounder a more careful dietician should have caught!)
There are many accounts, both new and old, of the lack of obesity in the populations consuming a “traditional”, pre-industrial diet (Lieberman, 2013; Pontzer, 2021; Pontzer et al., 2018; Price, 1938; Trowell & Burkitt, 1981). It’s indisputable that prior to the modern, industrial age, obesity was a very rare condition, as were the rest of the chronic diseases.
There are few accounts more specific that Weston Price’s from the 1930s:
“The problem of estimating… the displacing foods used by the various primitive races, is similar in many respects to estimating these qualities in the foods used in our modern… civilizations, except that modern commerce has transported usually only the foods that will keep well. These include chiefly white flour, sugar, polished rice, vegetable fats and canned goods.” (Price, 1938)
One population that has garnered a lot of attention recently has been the Tsimane, who live in the Bolivian Amazonian jungle. The cardiology profession has a love affair with the Tsimane, since they are under the impression that the apparently low LDL levels of these people contributes to their low rates of heart disease, and all the other chronic diseases (Kaplan et al., 2017).
One of the diseases that the Tsimane avoid is obesity. Well, until recently. Three papers looked at the increase incidence of obesity in the Tsimane, and the conclusion they come to is that “market foods” seem be the driver of this process, as sugar and cooking oil consumption increases (Bethancourt et al., 2019; Kraft et al., 2018; Rosinger et al., 2013).
The most recent of the three, building upon prior work, concludes:
“We found that household use of cooking oil was positively associated with female BMI… Males were more likely than females to consume less than their estimated energy needs and report low fat intake, which may be one reason we did not observe strong relationships between oil consumption and adiposity measures in men.” (Bethancourt et al., 2019)
Men’s increase in BMI was also lower than that of women.
“By 2010, the number of households using cooking oil doubled from 14.5% to 30.2%... This translated into household calories from oil increasing from 2.3% to 5.2% of calorie (Table 2; Figure 3C).” (Bethancourt et al., 2019)
5.2% of calories, depending on the type of seed oil, brings the Tsimane to the level of seed oil consumption seen in the U.S. in the early 1960s (Blasbalg et al., 2011), a period at which some investigators were starting to notice a link between seed oil consumption and weight gain.
“Adipose tissue linoleic acid rose in men on the experimental diet from 11% of total fatty acid at time zero to 32% at 5 years. The rise could be fitted to an exponential function with a half-time of 680 days. The rate of rise during the 1st year was correlated negatively with initial body weight and positively with weight gain; the influence of adherence to the diet was much less pronounced.” (Dayton et al., 1966)
While the experience of increased body weight varied in such studies—see (Woodhill et al., 1978) for a much later study that did not see a difference in weight gain—it’s clear that the effect likely starts from a low level of seed oil consumption, like that seen in the Tsimane or the U.S. population in (Blasbalg et al., 2011).
One of the most interesting epidemiological studies comes from three half-skeptics on the harmfulness of seed oils (Mozaffarian et al., 2011). I wrote about this recently (Goodrich, 2021), and so will just note the dramatic difference they found in weight gain from potato foods containing seed oils to those that don’t. They found that potatoes fried in seed oils were, by far, the most fattening food they found in a large, prestigious U.S. dataset.
While I was quite pleased to have noticed this, it turns out I wasn’t the first:
“Drs. Mozaffarian et al have extended the important observation that the selective nutrient content of foods may be important determinants of weight gain. However, the authors missed an opportunity to critically examine the differences in the nutrient composition of french fries and chips compared to other potatoes. French fries had a 6-fold greater effect in weight gain and also differ from potatoes by having much greater omega-6 linoleic acid (7 gm/100 gm) vs. 0.03 gm/100 gm for baked potatoes (USDA Nutrient Database V 23). Potato chips have 13.4 gm of omega-6 linoleic acid/100 gm. Linoleic acid has been identified as a precursor for endogenous cannabinoids that are critical mediators of appetite and, in excess impair satiety and induce weight gain via mechanisms similar to pharmacological cannabinoids.” (Mozaffarian et al., 2011 J. Hibbeln, comment on online version)
Hibbeln was the senior author of the study with which we opened this discussion, “Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity” (A. Alvheim et al., 2014).
Conclusion
There is thus clear and consistent evidence in both animals and humans to think that LA is causative in obesity, given its role as a precursor to the endocannabinoid system that clearly plays a major role in energy homeostasis and thus obesity (Hawkins & Horvath, 2017). While it is clearly not an acute effect in humans as it is in animal models, given the longer life expectancy of humans it’s not surprising that we have a greater tolerance for insult.
Essentially, we are eating a diet that is giving us the munchies, the effect that marijuana has on those that consume it. And the foods desired under stimulation of the ECS are sugar and starch (Koch, 2001; Simiand et al., 1998). As Stephan pointed out in 2012, that’s largely what we are eating more of (S. Guyenet, 2012).
While it wasn’t the intention of this effort, I also think the evidence for the Food Reward Hypothesis is pretty indisputable. It goes hand-in-hand with the LA hypothesis for obesity, as dietary LA appears to be the primary mechanism behind the process of food reward and hyperpalatibility.
But, as they say, that’s not all. In Part 2 we’ll look at a further confirmation of the role of LA and the ECS in obesity, and at a fundamental underlying mechanism that may elucidate exactly what is going on.
Part 2: Roux-en-Y Gastric Bypass and 4-Hydroxynonenal
In which we will go over some specific mechanisms that may explain exactly how this happens…
Original post November 18, 2021.
P.S. Per an excellent suggestion from Jeff Nobbs, I included the N6:N3 ratio for the two studies from Kevin Hall, (Hall et al., 2019; 2021) in the graphics included for those studies. The data is the same, just reformatted, with the n-6, n-3, and ratio now shown. This makes the relationship much more obvious.
References
Abbott Laboratories, Inc. (2020, November 9). Ensure® Original Vanilla Meal Replacement Shake [Advertisement]. Ensure®: Strength and Energy. https://ensure.com/nutrition-products/ensure-original/vanilla-shake
Alvheim, A. R., Malde, M. K., Osei‐Hyiaman, D., Hong, Y. H., Pawlosky, R. J., Madsen, L., Kristiansen, K., Frøyland, L., & Hibbeln, J. R. (2012). Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity, 20(10), 1984–1994. https://doi.org/10.1038/oby.2012.38
Alvheim, A. R., Torstensen, B. E., Lin, Y. H., Lillefosse, H. H., Lock, E.-J., Madsen, L., Hibbeln, J. R., & Malde, M. K. (2013). Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice. The British Journal of Nutrition, 109(8), 1508–1517. https://doi.org/10.1017/S0007114512003364
Alvheim, A., Torstensen, B. E., Lin, Y. H., Lillefosse, H. H., Lock, E.-J., Madsen, L., Frøyland, L., Hibbeln, J. R., & Malde, M. K. (2014). Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids, 49(1), 59–69. https://doi.org/10.1007/s11745-013-3842-y
Athanasiou, A., Clarke, A. B., Turner, A. E., Kumaran, N. M., Vakilpour, S., Smith, P. A., Bagiokou, D., Bradshaw, T. D., Westwell, A. D., Fang, L., Lobo, D. N., Constantinescu, C. S., Calabrese, V., Loesch, A., Alexander, S. P. H., Clothier, R. H., Kendall, D. A., & Bates, T. E. (2007). Cannabinoid receptor agonists are mitochondrial inhibitors: A unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochemical and Biophysical Research Communications, 364(1), 131–137. https://doi.org/10.1016/j.bbrc.2007.09.107
Avalos, B., Argueta, D. A., Perez, P. A., Wiley, M., Wood, C., & DiPatrizio, N. V. (2020). Cannabinoid CB1 Receptors in the Intestinal Epithelium Are Required for Acute Western-Diet Preferences in Mice. Nutrients, 12(9). https://doi.org/10.3390/nu12092874
Baur, F. J., & Brown, J. B. (1945). The Fatty Acids of Corn Oil. Journal of the American Chemical Society, 67(11), 1899–1900. https://doi.org/10.1021/ja01227a007
Beal, J. E., Olson, R., Lefkowitz, L., Laubenstein, L., Bellman, P., Yangco, B., Morales, J. O., Murphy, R., Powderly, W., Plasse, T. F., Mosdell, K. W., & Shepard, K. V. (1997). Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. Journal of Pain and Symptom Management, 14(1), 7–14. https://doi.org/10.1016/S0885-3924(97)00038-9
Bethancourt, H. J., Leonard, W. R., Tanner, S., Schultz, A. F., & Rosinger, A. Y. (2019). Longitudinal Changes in Measures of Body Fat and Diet Among Adult Tsimane’ Forager-Horticulturalists of Bolivia, 2002-2010. Obesity, 27(8), 1347–1359. https://doi.org/10.1002/oby.22556
Blasbalg, T. L., Hibbeln, J. R., Ramsden, C. E., Majchrzak, S. F., & Rawlings, R. R. (2011). Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American Journal of Clinical Nutrition, 93(5), 950–962. https://doi.org/10.3945/ajcn.110.006643
Blüher, M., Engeli, S., Klöting, N., Berndt, J., Fasshauer, M., Bátkai, S., Pacher, P., Schön, M. R., Jordan, J., & Stumvoll, M. (2006). Dysregulation of the Peripheral and Adipose Tissue Endocannabinoid System in Human Abdominal Obesity. Diabetes, 55(11), 3053–3060. https://doi.org/10.2337/db06-0812
Bronander, K. A., & Bloch, M. J. (2007). Potential role of the endocannabinoid receptor antagonist rimonabant in the management of cardiometabolic risk: A narrative review of available data. Vascular Health and Risk Management, 3(2), 181–190. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1994026/
Brown, J. D., Karimian Azari, E., & Ayala, J. E. (2017). Oleoylethanolamide: A fat ally in the fight against obesity. Physiology & Behavior, 176, 50–58. https://doi.org/10.1016/j.physbeh.2017.02.034
Côté, M., Matias, I., Lemieux, I., Petrosino, S., Alméras, N., Després, J.-P., & Di Marzo, V. (2007). Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. International Journal of Obesity, 31(4), 692–699. https://doi.org/10.1038/sj.ijo.0803539
Dayton, S., Hashimoto, S., Dixon, W., & Pearce, M. L. (1966). Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. Journal of Lipid Research, 7(1), 103–111. https://www.jlr.org/article/S0022-2275(20)39591-2/pdf
Depner, C. M., Philbrick, K. A., & Jump, D. B. (2013). Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(-/-) mouse model of western diet-induced nonalcoholic steatohepatitis. The Journal of Nutrition, 143(3), 315–323. https://doi.org/10.3945/jn.112.171322
DiPatrizio, N. V., Astarita, G., Schwartz, G., Li, X., & Piomelli, D. (2011). Endocannabinoid signal in the gut controls dietary fat intake. Proceedings of the National Academy of Sciences of the United States of America, 108(31), 12904–12908. https://doi.org/10.1073/pnas.1104675108
DiPatrizio, N. V., Igarashi, M., Narayanaswami, V., Murray, C., Gancayco, J., Russell, A., Jung, K.-M., & Piomelli, D. (2015). Fasting stimulates 2-AG biosynthesis in the small intestine: Role of cholinergic pathways. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 309(8), R805–R813. https://doi.org/10.1152/ajpregu.00239.2015
DiPatrizio, N. V., Joslin, A., Jung, K.-M., & Piomelli, D. (2013). Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. The FASEB Journal, 27(6), 2513–2520. https://doi.org/10.1096/fj.13-227587
Dobromylskyj, P. (2012, September 17). Hyperlipid: Protons: Linoleic acid in the hypothalamus. Hyperlipid. http://high-fat-nutrition.blogspot.com/2012/09/protons-linoleic-acid-in-hypothalamus.html
Enos, R. T., Velázquez, K. T., McClellan, J. L., Cranford, T. L., Walla, M. D., & Murphy, E. A. (2014). Reducing the dietary omega-6:omega-3 utilizing α-linolenic acid; not a sufficient therapy for attenuating high-fat-diet-induced obesity development nor related detrimental metabolic and adipose tissue inflammatory outcomes. PloS One, 9(4), e94897–e94897. https://doi.org/10.1371/journal.pone.0094897
Flint, A., Helt, B., Raben, A., Toubro, S., & Astrup, A. (2003). Effects of Different Dietary Fat Types on Postprandial Appetite and Energy Expenditure. Obesity Research, 11(12), 1449–1455. https://doi.org/10.1038/oby.2003.194
Fu, J., Oveisi, F., Gaetani, S., Lin, E., & Piomelli, D. (2005). Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology, 48(8), 1147–1153. https://doi.org/10.1016/j.neuropharm.2005.02.013
Garaulet, M., Pérez-Llamas, F., Pérez-Ayala, M., Martínez, P., de Medina, F. S., Tebar, F. J., & Zamora, S. (2001). Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: Relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. The American Journal of Clinical Nutrition, 74(5), 585–591. https://doi.org/10.1093/ajcn/74.5.585
Garg, M. L., Keelan, M., Thomson, A. B., & Clandinin, M. T. (1992). Desaturation of linoleic acid in the small bowel is increased by short-term fasting and by dietary content of linoleic acid. Biochimica Et Biophysica Acta, 1126(1), 17–25. https://doi.org/10.1016/0005-2760(92)90211-d
Gatta-Cherifi, B., Matias, I., Vallée, M., Tabarin, A., Marsicano, G., Piazza, P. V., & Cota, D. (2012). Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. International Journal of Obesity, 36(6), 880–885. https://doi.org/10.1038/ijo.2011.165
Ghosh, S., O’Connell, J. F., Carlson, O. D., González‐Mariscal, I., Kim, Y., Moaddel, R., Ghosh, P., & Egan, J. M. (2019). Linoleic acid in diets of mice increases total endocannabinoid levels in bowel and liver: Modification by dietary glucose. Obesity Science & Practice, 5(4), 383–394. https://doi.org/10.1002/osp4.344
Ghosh, S., Qi, D., An, D., Pulinilkunnil, T., Abrahani, A., Kuo, K.-H., Wambolt, R. B., Allard, M., Innis, S. M., & Rodrigues, B. (2004). Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA. American Journal of Physiology. Heart and Circulatory Physiology, 287(6), H2518-2527. https://doi.org/10.1152/ajpheart.00480.2004
Goodrich, T. (2010, August 18). “Diverticulitis”: My Story [Blog]. Yelling Stop. http://yelling-stop.blogspot.com/2010/08/diverticulitis-my-story.html
Goodrich, T. (2011, November 19). Linoleic Acid, Fat Rats in Labs, and Fat Humans. Yelling Stop. http://yelling-stop.blogspot.com/2011/11/linoleic-acid-fat-rats-in-labs-and-fat.html
Goodrich, T. (2018, June 28). What’s Worse—Carbs or Seed Oils? Understanding a High-PUFA Diet. Yelling Stop. http://yelling-stop.blogspot.com/2018/06/whats-worsecarbs-or-seed-oils.html
Goodrich, T. (2021, October 19). What Is The Most Fattening Food? [Blog]. Yelling Stop. https://yelling-stop.blogspot.com/2021/10/whats-most-fattening-food.html
Gorelick, D. A., Goodwin, R. S., Schwilke, E., Schwope, D. M., Darwin, W. D., Kelly, D. L., McMahon, R. P., Liu, F., Ortemann-Renon, C., Bonnet, D., & Huestis, M. A. (2011). Antagonist-Elicited Cannabis Withdrawal in Humans. Journal of Clinical Psychopharmacology, 31(5), 603–612. https://doi.org/10.1097/JCP.0b013e31822befc1
Grosshans, M., Schwarz, E., Bumb, J. M., Schaefer, C., Rohleder, C., Vollmert, C., Vollstädt-Klein, S., Tost, H., Meyer-Lindenberg, A., Kiefer, F., & Leweke, F. M. (2014). Oleoylethanolamide and Human Neural Responses to Food Stimuli in Obesity. JAMA Psychiatry, 71(11), 1254. https://doi.org/10.1001/jamapsychiatry.2014.1215
Guijarro, A., Fu, J., Astarita, G., & Piomelli, D. (2010). CD36 gene deletion decreases oleoylethanolamide levels in small intestine of free-feeding mice. Pharmacological Research, 61(1), 27–33. https://doi.org/10.1016/j.phrs.2009.09.003
Guyenet, S. (2012, September 14). Whole Health Source: More Thoughts on Macronutrient Trends. Whole Health Source. http://wholehealthsource.blogspot.com/2012/09/more-thoughts-on-macronutrient-trends.html
Guyenet, S. J. (2011a, August 21). Seed Oils and Body Fatness—A Problematic Revisit [Blog]. Whole Health Source. http://wholehealthsource.blogspot.com/2011/08/seed-oils-and-body-fatness-problematic.html
Guyenet, S. J. (2011b, September 24). Humans on a Cafeteria Diet [Blog]. Whole Health Source. http://wholehealthsource.blogspot.com/2011/09/humans-on-cafeteria-diet.html
Guyenet, S. J. (2011c, October 1). Whole Health Source: The Case for the Food Reward Hypothesis of Obesity, Part I. Whole Health Source. http://wholehealthsource.blogspot.com/2011/10/case-for-food-reward-hypothesis-of.html
Guyenet, S. J., & Carlson, S. E. (2015). Increase in Adipose Tissue Linoleic Acid of US Adults in the Last Half Century. Advances in Nutrition, 6(6), 660–664. https://doi.org/10.3945/an.115.009944
Guyenet, S. J., & Schwartz, M. W. (2012). Regulation of Food Intake, Energy Balance, and Body Fat Mass: Implications for the Pathogenesis and Treatment of Obesity. The Journal of Clinical Endocrinology & Metabolism, 97(3), 745–755. https://doi.org/10.1210/jc.2011-2525
Hall, K. D. (2021, May 6). Energy intake figures for Hall and al. 2021 [Personal communication].
Hall, K. D., Ayuketah, A., Brychta, R., Cai, H., Cassimatis, T., Chen, K. Y., Chung, S. T., Costa, E., Courville, A., Darcey, V., Fletcher, L. A., Forde, C. G., Gharib, A. M., Guo, J., Howard, R., Joseph, P. V., McGehee, S., Ouwerkerk, R., Raisinger, K., … Zhou, M. (2019). Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metabolism, 30(1), 67-77.e3. https://doi.org/10.1016/j.cmet.2019.05.008
Hall, K. D., Guo, J., Courville, A. B., Boring, J., Brychta, R., Chen, K. Y., Darcey, V., Forde, C. G., Gharib, A. M., Gallagher, I., Howard, R., Joseph, P. V., Milley, L., Ouwerkerk, R., Raisinger, K., Rozga, I., Schick, A., Stagliano, M., Torres, S., … Chung, S. T. (2021). Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nature Medicine, 27(2), 344–353. https://doi.org/10.1038/s41591-020-01209-1
Han, W., Tellez, L. A., Perkins, M. H., Perez, I. O., Qu, T., Ferreira, J., Ferreira, T. L., Quinn, D., Liu, Z.-W., Gao, X.-B., Kaelberer, M. M., Bohórquez, D. V., Shammah-Lagnado, S. J., Lartigue, G. de, & Araujo, I. E. de. (2018). A Neural Circuit for Gut-Induced Reward. Cell, 175(3), 665-678.e23. https://doi.org/10.1016/j.cell.2018.08.049
Hawkins, M. N., & Horvath, T. L. (2017). Cannabis in fat: High hopes to treat obesity. The Journal of Clinical Investigation, 127(11), 3918–3920. https://doi.org/10.1172/JCI97042
Hodge, A. M., English, D. R., O’Dea, K., Sinclair, A. J., Makrides, M., Gibson, R. A., & Giles, G. G. (2007). Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: Interpreting the role of linoleic acid. The American Journal of Clinical Nutrition, 86(1), 189–197. https://doi.org/10.1093/ajcn/86.1.189
Huestis, M. A., Boyd, S. J., Heishman, S. J., Preston, K. L., Bonnet, D., Le Fur, G., & Gorelick, D. A. (2007). Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology, 194(4), 505–515. https://doi.org/10.1007/s00213-007-0861-5
Igarashi, M., DiPatrizio, N. V., Narayanaswami, V., & Piomelli, D. (2015). Feeding-induced oleoylethanolamide mobilization is disrupted in the gut of diet-induced obese rodents. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1851(9), 1218–1226. https://doi.org/10.1016/j.bbalip.2015.05.006
Jbilo, O., Ravinet‐Trillou, C., Arnone, M., Buisson, I., Bribes, E., Péleraux, A., Pénarier, G., Soubrié, P., Fur, G. L., Galiègue, S., & Casellas, P. (2005). The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. The FASEB Journal, 19(11), 1567–1569. https://doi.org/10.1096/fj.04-3177fje
Joosten, M. M., Balvers, M. G., Verhoeckx, K. C., Hendriks, H. F., & Witkamp, R. F. (2010). Plasma anandamide and other N-acylethanolamines are correlated with their corresponding free fatty acid levels under both fasting and non-fasting conditions in women. Nutrition & Metabolism, 7(1), 49. https://doi.org/10.1186/1743-7075-7-49
Justinová, Z., Yasar, S., Redhi, G. H., & Goldberg, S. R. (2011). The Endogenous Cannabinoid 2-Arachidonoylglycerol Is Intravenously Self-Administered by Squirrel Monkeys. Journal of Neuroscience, 31(19), 7043–7048. https://doi.org/10.1523/JNEUROSCI.6058-10.2011
Kaplan, H., Thompson, R. C., Trumble, B. C., Wann, L. S., Allam, A. H., Beheim, B., Frohlich, B., Sutherland, M. L., Sutherland, J. D., Stieglitz, J., Rodriguez, D. E., Michalik, D. E., Rowan, C. J., Lombardi, G. P., Bedi, R., Garcia, A. R., Min, J. K., Narula, J., Finch, C. E., … Thomas, G. S. (2017). Coronary atherosclerosis in indigenous South American Tsimane: A cross-sectional cohort study. The Lancet, 389(10080), 1730–1739. https://doi.org/10.1016/S0140-6736(17)30752-3
Karlsson, M., Mårild, S., Brandberg, J., Lönn, L., Friberg, P., & Strandvik, B. (2006). Serum Phospholipid Fatty Acids, Adipose Tissue, and Metabolic Markers in Obese Adolescents. Obesity, 14(11), 1931–1939. https://doi.org/10.1038/oby.2006.225
Kaviani, S., & Cooper, J. A. (2017). Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. European Journal of Clinical Nutrition, 71(10), 1154–1165. https://doi.org/10.1038/ejcn.2016.250
Kirkham, T. C., & Williams, C. M. (2001). Endogenous cannabinoids and appetite. Nutrition Research Reviews, 14(1), 65–86. https://doi.org/10.1079/NRR200118
Kirkham, T. C., Williams, C. M., Fezza, F., & Marzo, V. D. (2002). Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. British Journal of Pharmacology, 136(4), 550–557. https://doi.org/10.1038/sj.bjp.0704767
Koch, J. E. (2001). Δ9-THC stimulates food intake in Lewis rats: Effects on chow, high-fat and sweet high-fat diets. Pharmacology, Biochemistry, and Behavior, 68(3), 539–543. https://doi.org/10.1016/s0091-3057(01)00467-1
Kraft, T. S., Stieglitz, J., Trumble, B. C., Martin, M., Kaplan, H., & Gurven, M. (2018). Nutrition transition in 2 lowland Bolivian subsistence populations. The American Journal of Clinical Nutrition, 108(6), 1183–1195. https://doi.org/10.1093/ajcn/nqy250
Laleh, P., Yaser, K., Abolfazl, B., Shahriar, A., Mohammad, A. J., Nazila, F., & Alireza, O. (2018). Oleoylethanolamide increases the expression of PPAR-Α and reduces appetite and body weight in obese people: A clinical trial. Appetite, 128, 44–49. https://doi.org/10.1016/j.appet.2018.05.129
Laleh, P., Yaser, K., & Alireza, O. (2019). Oleoylethanolamide: A novel pharmaceutical agent in the management of obesity-an updated review. Journal of Cellular Physiology, 234(6), 7893–7902. https://doi.org/10.1002/jcp.27913
Lieberman, D. (2013). The Story of the Human Body: Evolution, Health, and Disease. Knopf Doubleday Publishing Group. https://www.penguinrandomhouse.com/books/206671/the-story-of-the-human-body-by-daniel-e-lieberman/
Lindenmeyer, C. C., & McCullough, A. J. (2018). The Natural History of Nonalcoholic Fatty Liver Disease—An Evolving View. Clinics in Liver Disease, 22(1), 11. https://doi.org/10.1016/j.cld.2017.08.003
Little, T. J., Cvijanovic, N., DiPatrizio, N. V., Argueta, D. A., Rayner, C. K., Feinle-Bisset, C., & Young, R. L. (2018). Plasma endocannabinoid levels in lean, overweight, and obese humans: Relationships to intestinal permeability markers, inflammation, and incretin secretion. American Journal of Physiology-Endocrinology and Metabolism, 315(4), E489–E495. https://doi.org/10.1152/ajpendo.00355.2017
Liu, T.-W. (2013). Changes in fatty acids profiles after three weeks of high-fat diet feeding in obesity-prone rats [Master’s Dissertation, University of Missouri--Columbia]. https://doi.org/10.32469/10355/44001
Maciejewska, D., Marlicz, W., Ryterska, K., Banaszczak, M., Jamioł-Milc, D., & Stachowska, E. (2018). Changes of the Fatty Acid Profile in Erythrocyte Membranes of Patients following 6-Month Dietary Intervention Aimed at the Regression of Nonalcoholic Fatty Liver Disease (NAFLD). Canadian Journal of Gastroenterology & Hepatology, 2018. https://doi.org/10.1155/2018/5856201
Maciejewska, D., Ossowski, P., Drozd, A., Ryterska, K., Jamioł-Milc, D., Banaszczak, M., Kaczorowska, M., Sabinicz, A., Raszeja-Wyszomirska, J., & Stachowska, E. (2015). Metabolites of arachidonic acid and linoleic acid in early stages of non-alcoholic fatty liver disease—A pilot study. Prostaglandins & Other Lipid Mediators, 121, 184–189. https://doi.org/10.1016/j.prostaglandins.2015.09.003
Mann, J. D., Faurot, K. R., MacIntosh, B., Palsson, O. S., Suchindran, C. M., Gaylord, S. A., Lynch, C., Johnston, A., Maiden, K., Barrow, D. A., Hibbeln, J. R., & Ramsden, C. E. (2018). A sixteen-week three-armed, randomized, controlled trial investigating clinical and biochemical effects of targeted alterations in dietary linoleic acid and n-3 EPA+DHA in adults with episodic migraine: Study protocol. Prostaglandins, Leukotrienes and Essential Fatty Acids, 128, 41–52. https://doi.org/10.1016/j.plefa.2017.11.002
Martin, D. (2015). TD.97366 Adjusted Fat Diet [Personal communication].
Mastinu, A., Pira, M., Pani, L., Pinna, G. A., & Lazzari, P. (2012). NESS038C6, a novel selective CB1 antagonist agent with anti-obesity activity and improved molecular profile. Behavioural Brain Research, 234(2), 192–204. https://doi.org/10.1016/j.bbr.2012.06.033
Matias, I., Petrosino, S., Racioppi, A., Capasso, R., Izzo, A. A., & Di Marzo, V. (2008). Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Molecular and Cellular Endocrinology, 286(1-2 Suppl 1), S66-78. https://doi.org/10.1016/j.mce.2008.01.026
Mennella, I., Savarese, M., Ferracane, R., Sacchi, R., & Vitaglione, P. (2015). Oleic acid content of a meal promotes oleoylethanolamide response and reduces subsequent energy intake in humans. Food & Function, 6(1), 203–209. https://doi.org/10.1039/C4FO00697F
Midtbø, L. K., Ibrahim, M. M., Myrmel, L. S., Aune, U. L., Alvheim, A. R., Liland, N. S., Torstensen, B. E., Rosenlund, G., Liaset, B., Brattelid, T., Kristiansen, K., & Madsen, L. (2013). Intake of farmed Atlantic salmon fed soybean oil increases insulin resistance and hepatic lipid accumulation in mice. PloS One, 8(1), e53094. https://doi.org/10.1371/journal.pone.0053094
Monteiro, C. A., Cannon, G., Moubarac, J.-C., Levy, R. B., Louzada, M. L. C., & Jaime, P. C. (2018). The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutrition, 21(1), 5–17. https://doi.org/10.1017/S1368980017000234
Mozaffarian, D., Hao, T., Rimm, E. B., Willett, W. C., & Hu, F. B. (2011). Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. New England Journal of Medicine, 364(25), 2392–2404. https://doi.org/10.1056/NEJMoa1014296
Naughton, S. S., Hanson, E. D., Mathai, M. L., & McAinch, A. J. (2018). The Acute Effect of Oleic- or Linoleic Acid-Containing Meals on Appetite and Metabolic Markers; A Pilot Study in Overweight or Obese Individuals. Nutrients, 10(10). https://doi.org/10.3390/nu10101376
Naughton, S. S., Mathai, M. L., Hryciw, D. H., & McAinch, A. J. (2013). Fatty Acid Modulation of the Endocannabinoid System and the Effect on Food Intake and Metabolism. International Journal of Endocrinology, 2013, 361895. https://doi.org/10.1155/2013/361895
Naughton, S. S., Mathai, M. L., Hryciw, D. H., & McAinch, A. J. (2016). Linoleic acid and the pathogenesis of obesity. Prostaglandins & Other Lipid Mediators, 125, 90–99. https://doi.org/10.1016/j.prostaglandins.2016.06.003
Nigam, P., Bhatt, S., Misra, A., Chadha, D. S., Vaidya, M., Dasgupta, J., & Pasha, Q. M. A. (2014). Effect of a 6-Month Intervention with Cooking Oils Containing a High Concentration of Monounsaturated Fatty Acids (Olive and Canola Oils) Compared with Control Oil in Male Asian Indians with Nonalcoholic Fatty Liver Disease. Diabetes Technology & Therapeutics, 16(4), 255–261. https://doi.org/10.1089/dia.2013.0178
NIH. (2018, September 11). Principal Investigators [Biography]. NIH Intramural Research Program. https://irp.nih.gov/pi/joseph-hibbeln
Osei-Hyiaman, D., DePetrillo, M., Pacher, P., Liu, J., Radaeva, S., Bátkai, S., Harvey-White, J., Mackie, K., Offertáler, L., Wang, L., & Kunos, G. (2005). Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. Journal of Clinical Investigation, 115(5), 1298–1305. https://doi.org/10.1172/JCI200523057
Pan, D. A., & Storlien, L. H. (1993). Dietary lipid profile is a determinant of tissue phospholipid fatty acid composition and rate of weight gain in rats. The Journal of Nutrition, 123(3), 512–519. https://doi.org/10.1093/jn/123.3.512
Payahoo, L., Khajebishak, Y., Alivand, M. R., Soleimanzade, H., Alipour, S., Barzegari, A., & Ostadrahimi, A. (2019). Investigation the effect of oleoylethanolamide supplementation on the abundance of Akkermansia muciniphila bacterium and the dietary intakes in people with obesity: A randomized clinical trial. Appetite, 141, 104301. https://doi.org/10.1016/j.appet.2019.05.032
Petersen, G., Sørensen, C., Schmid, P. C., Artmann, A., Tang-Christensen, M., Hansen, S. H., Larsen, P. J., Schmid, H. H. O., & Hansen, H. S. (2006). Intestinal levels of anandamide and oleoylethanolamide in food-deprived rats are regulated through their precursors. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1761(2), 143–150. https://doi.org/10.1016/j.bbalip.2005.12.011
Pittalwala, I. (2017, October 2). GM soybean oil causes less obesity and insulin resistance but is harmful to liver function: Mouse study compares Plenish to conventional soybean, coconut, and olive oils [News]. ScienceDaily. https://www.sciencedaily.com/releases/2017/10/171002084828.htm
Pontzer, H. (2021). Burn: New Research Blows the Lid Off How We Really Burn Calories, Lose Weight, and Stay Healthy. Penguin. https://www.google.com/books/edition/Burn/4APyDwAAQBAJ?hl=en
Pontzer, H., Wood, B. M., & Raichlen, D. A. (2018). Hunter-gatherers as models in public health. Obesity Reviews, 19(S1), 24–35. https://doi.org/10.1111/obr.12785
Price, W. (1938). Nutrition and Physical Degeneration. http://gutenberg.net.au/ebooks02/0200251h.html
Ramsden, C. E., Ringel, A., Feldstein, A. E., Taha, A. Y., MacIntosh, B. A., Hibbeln, J. R., Majchrzak-Hong, S. F., Faurot, K. R., Rapoport, S. I., Cheon, Y., Chung, Y.-M., Berk, M., & Douglas Mann, J. (2012). Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins, Leukotrienes and Essential Fatty Acids, 87(4), 135–141. https://doi.org/10.1016/j.plefa.2012.08.004
Ravinet Trillou, C., Arnone, M., Delgorge, C., Gonalons, N., Keane, P., Maffrand, J.-P., & Soubrié, P. (2003). Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 284(2), R345–R353. https://doi.org/10.1152/ajpregu.00545.2002
Ravinet Trillou, C., Delgorge, C., Menet, C., Arnone, M., & Soubrié, P. (2004). CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. International Journal of Obesity, 28(4), 640–648. https://doi.org/10.1038/sj.ijo.0802583
Research Diets, Inc. (2006a). D12450B Formula [Advertisement]. D12450B. https://researchdiets.com/formulas/d12450b
Research Diets, Inc. (2006b). D12492 Formula [Advertisement]. D12492. https://researchdiets.com/formulas/d12492
Research Diets, Inc. (2009). High Fat Diets for Diet-Induced Obesity Models [Advertisement]. https://labanimal.co.kr/product/pdf/Obesity.pdf
Rogge, M. M. (2009). The Role of Impaired Mitochondrial Lipid Oxidation in Obesity: Biological Research for Nursing, 10(4), 356–373. https://doi.org/10.1177/1099800408329408
Romano, A., Coccurello, R., Giacovazzo, G., Bedse, G., Moles, A., & Gaetani, S. (2014). Oleoylethanolamide: A novel potential pharmacological alternative to cannabinoid antagonists for the control of appetite. BioMed Research International, 2014, 203425. https://doi.org/10.1155/2014/203425
Rosinger, A., Tanner, S., Leonard, W. R., & TAPS Bolivia Research Team. (2013). Precursors to overnutrition: The effects of household market food expenditures on measures of body composition among Tsimane’ adults in lowland Bolivia. Social Science & Medicine (1982), 92, 53–60. https://doi.org/10.1016/j.socscimed.2013.05.022
Rossmeisl, M., Jilkova, Z. M., Kuda, O., Jelenik, T., Medrikova, D., Stankova, B., Kristinsson, B., Haraldsson, G. G., Svensen, H., Stoknes, I., Sjövall, P., Magnusson, Y., Balvers, M. G. J., Verhoeckx, K. C. M., Tvrzicka, E., Bryhn, M., & Kopecky, J. (2012). Metabolic Effects of n-3 PUFA as Phospholipids Are Superior to Triglycerides in Mice Fed a High-Fat Diet: Possible Role of Endocannabinoids. PLOS ONE, 7(6), e38834. https://doi.org/10.1371/journal.pone.0038834
Saito, E., Okada, T., Abe, Y., Kuromori, Y., Miyashita, M., Iwata, F., Hara, M., Ayusawa, M., Mugishima, H., & Kitamura, Y. (2011). Docosahexaenoic Acid Content in Plasma Phospholipids and Desaturase Indices in Obese Children. Journal of Atherosclerosis and Thrombosis, advpub, 1102040344–1102040344. https://doi.org/10.5551/jat.6270
Schwartz, G. J., Fu, J., Astarita, G., Li, X., Gaetani, S., Campolongo, P., Cuomo, V., & Piomelli, D. (2008). The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metabolism, 8(4), 281–288. https://doi.org/10.1016/j.cmet.2008.08.005
Siegmund, S. V., Qian, T., Minicis, S. de, Harvey‐White, J., Kunos, G., Vinod, K. Y., Hungund, B., & Schwabe, R. F. (2007). The endocannabinoid 2-arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. The FASEB Journal, 21(11), 2798–2806. https://doi.org/10.1096/fj.06-7717com
Sigma-Aldrich, Inc. (2020, November 11). Intralipid I141 [Advertisement]. Sigma-Aldrich, Inc. https://www.sigmaaldrich.com/catalog/product/sigma/i141
Simiand, J., Keane, M., Keane, P. E., & Soubrié, P. (1998). SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behavioural Pharmacology, 9(2), 179–181. https://pubmed.ncbi.nlm.nih.gov/10065938/
Sipe, J. C., Scott, T. M., Murray, S., Harismendy, O., Simon, G. M., Cravatt, B. F., & Waalen, J. (2010). Biomarkers of Endocannabinoid System Activation in Severe Obesity. PLoS ONE, 5(1). https://doi.org/10.1371/journal.pone.0008792
Sipe, J. C., Waalen, J., Gerber, A., & Beutler, E. (2005). Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). International Journal of Obesity (2005), 29(7), 755–759. https://doi.org/10.1038/sj.ijo.0802954
Soria-Gómez, E., Matias, I., Rueda-Orozco, P. E., Cisneros, M., Petrosino, S., Navarro, L., Marzo, V. D., & Prospéro-García, O. (2007). Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. British Journal of Pharmacology, 151(7), 1109. https://doi.org/10.1038/sj.bjp.0707313
Strassburg, K., Esser, D., Vreeken, R. J., Hankemeier, T., Müller, M., Duynhoven, J. van, Golde, J. van, Dijk, S. J. van, Afman, L. A., & Jacobs, D. M. (2014). Postprandial fatty acid specific changes in circulating oxylipins in lean and obese men after high-fat challenge tests. Molecular Nutrition & Food Research, 58(3), 591–600. https://doi.org/10.1002/mnfr.201300321
Tellez, L. A., Medina, S., Han, W., Ferreira, J. G., Licona-Limón, P., Ren, X., Lam, T. T., Schwartz, G. J., & de Araujo, I. E. (2013). A gut lipid messenger links excess dietary fat to dopamine deficiency. Science (New York, N.Y.), 341(6147), 800–802. https://doi.org/10.1126/science.1239275
Tincopa, M. A. (2020). Diagnostic and interventional circulating biomarkers in nonalcoholic steatohepatitis. Endocrinology, Diabetes & Metabolism, 3(4), e00177. https://doi.org/10.1002/edm2.177
Trowell, H. C., & Burkitt, D. P. (1981). Western Diseases, Their Emergence and Prevention. Harvard University Press. https://www.hup.harvard.edu/catalog.php?isbn=9780674950207
Tsurutani, Y., Inoue, K., Sugisawa, C., Saito, J., Omura, M., & Nishikawa, T. (2018). Increased Serum Dihomo-γ-linolenic Acid Levels Are Associated with Obesity, Body Fat Accumulation, and Insulin Resistance in Japanese Patients with Type 2 Diabetes. Internal Medicine, 57(20), 2929–2935. https://doi.org/10.2169/internalmedicine.0816-18
Tutunchi, H., Ostadrahimi, A., Saghafi‐Asl, M., & Maleki, V. (2019). The effects of oleoylethanolamide, an endogenous PPAR-α agonist, on risk factors for NAFLD: A systematic review. Obesity Reviews, 20(7), 1057–1069. https://doi.org/10.1111/obr.12853
Tutunchi, H., Saghafi‐Asl, M., & Ostadrahimi, A. (2020). A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-α, on the management and prevention of obesity. Clinical and Experimental Pharmacology and Physiology, 47(4), 543–552. https://doi.org/10.1111/1440-1681.13238
U.S. National Library of Medicine. (2017, September 15). Dronabinol: MedlinePlus Drug Information [Reference]. MedlinePlus. https://medlineplus.gov/druginfo/meds/a607054.html
Van Name, M. A., Chick, J. M., Savoye, M., Pierpont, B., Galuppo, B., Feldstein, A., & Santoro, N. (2019). Effect of a Low n6/n3 PUFA Diet on Intrahepatic Fat Content in Obese Adolescents. Diabetes, 68(Supplement 1). https://doi.org/10.2337/db19-772-P
Van Name, M. A., Savoye, M., Chick, J. M., Galuppo, B. T., Feldstein, A. E., Pierpont, B., Johnson, C., Shabanova, V., Ekong, U., Valentino, P. L., Kim, G., Caprio, S., & Santoro, N. (2020). A Low ω-6 to ω-3 PUFA Ratio (n–6:n–3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. The Journal of Nutrition, 150(9), 2314–2321. https://doi.org/10.1093/jn/nxaa183
Walker, O. S., Gurm, H., Sharma, R., Verma, N., May, L. L., & Raha, S. (2021). Delta-9-tetrahydrocannabinol inhibits invasion of HTR8/SVneo human extravillous trophoblast cells and negatively impacts mitochondrial function. Scientific Reports, 11(1), 4029. https://doi.org/10.1038/s41598-021-83563-9
Wang, H., Treadway, T., Covey, D. P., Cheer, J. F., & Lupica, C. R. (2015). Cocaine-Induced Endocannabinoid Mobilization in the Ventral Tegmental Area. Cell Reports, 12(12), 1997–2008. https://doi.org/10.1016/j.celrep.2015.08.041
Williams, C. M., & Kirkham, T. C. (2002). Observational analysis of feeding induced by Δ9-THC and anandamide. Physiology & Behavior, 76(2), 241–250. https://doi.org/10.1016/S0031-9384(02)00725-4
Witkamp, R. F. (2018). The role of fatty acids and their endocannabinoid-like derivatives in the molecular regulation of appetite. Molecular Aspects of Medicine, 64, 45–67. https://doi.org/10.1016/j.mam.2018.01.002
Woodhill, J. M., Palmer, A. J., Leelarthaepin, B., McGilchrist, C., & Blacket, R. B. (1978). Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Advances in Experimental Medicine and Biology, 109, 317–330. https://doi.org/10.1007/978-1-4684-0967-3_18
Woudstra, T. D., Drozdowski, L. A., Wild, G. E., Clandinin, M. T., Agellon, L. B., & Thomson, A. B. R. (2004). An isocaloric PUFA diet enhances lipid uptake and weight gain in aging rats. Lipids, 39(4), 343–354. https://doi.org/10.1007/s11745-004-1238-y
Yagin, N. L., Hajjarzadeh, S., Aliasgharzadeh, S., Aliasgari, F., & Mahdavi, R. (2020). The association of dietary patterns with endocannabinoids levels in overweight and obese women. Lipids in Health and Disease, 19(1), 161. https://doi.org/10.1186/s12944-020-01341-4











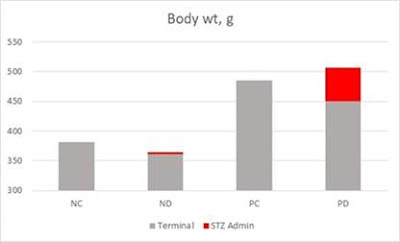





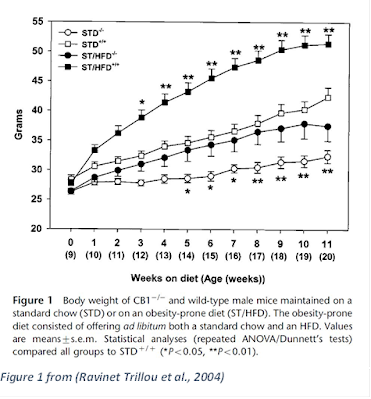


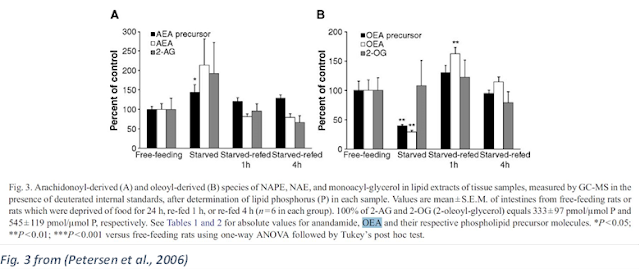




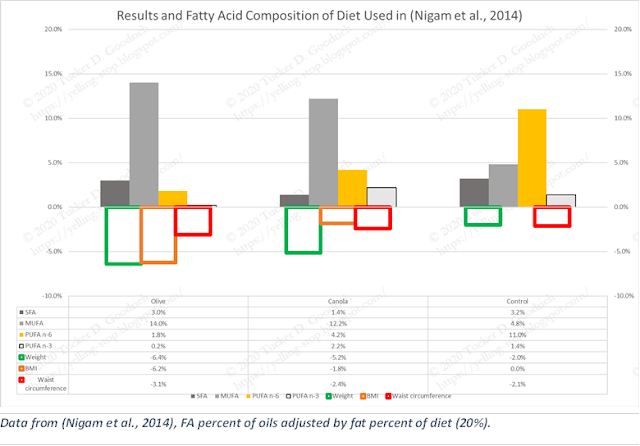

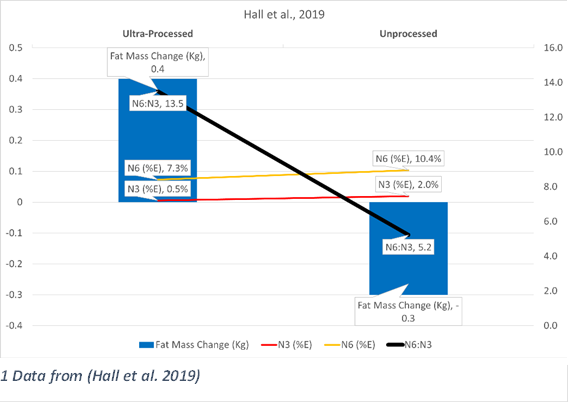





This is an amazing amount of work. Bravo!