Inflammation LOWERS Omega-6 Fats in the Body.
This is an excellent study showing a cause-and-effect, inverse relationship between disease status and linoleic acid.
Is Ω-6 protective against disease?
I've commented many times that Ω-6 level in serum isn't a good guide to
Diet (Kuriki, et al., 2003)
Disease status.
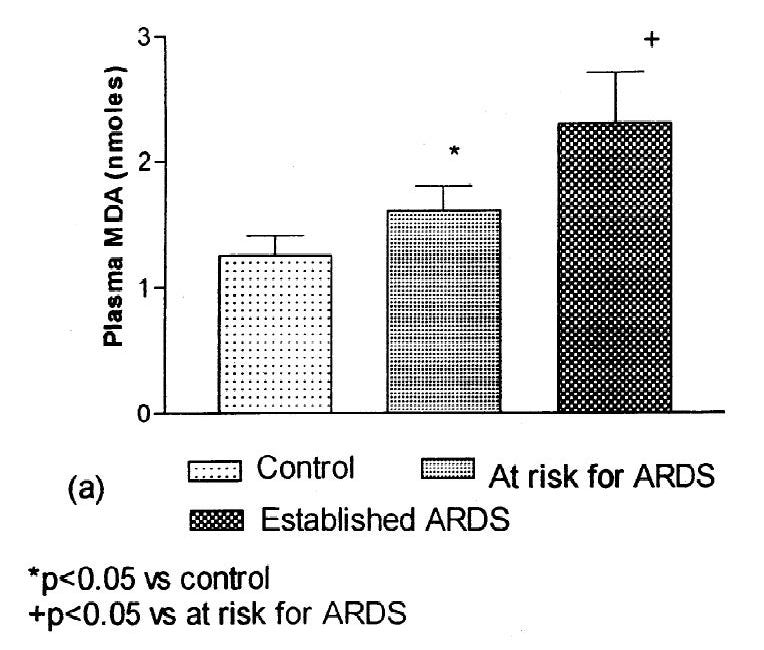
I've used the example of ARDS, where Ω-6 level is seen to decline as the disease progresses (Quinlan et al., 1996; Goodrich, 2020).
Does Consumption of Omega-6 Seed Oils Worsen ARDS and COVID-19?
1. Sometimes you just get lucky. Several years ago [1.01] I came across the following paper [1.02]: While I have only been able to access the abstract [got it, see PS at end], it's pretty telling of something I've seen implied in a number of different disease processes:
And I've suggested that increased levels of Ω-6 in, say, type 2 diabetes (T2DM), doesn't show that it prevents T2DM, but rather that it is decreased during T2DM.
Specifically, Hodge et al. found a positive relationship between dietary Ω-6, but an inverse relationship to plasma phospholipids.
“Plasma phospholipid linoleic acid was inversely, and dietary linoleic acid was positively, associated with diabetes risk. Persons who developed diabetes had lower plasma phospholipid linoleic acid proportions for each quintile of linoleic acid intake than did persons without diabetes.” (Hodge et al., 2007)
Similarly, (Zong et al., 2019) found that, “Total n-6 PUFAs were associated with a higher risk for type 2 diabetes in the age-adjusted model…”, only after some rather extravagant ‘adjustments’ were they able to correct this troubling finding. Co-authors included Walter Willett and Frank Hu of Harvard, and was funded, in part, by the Unilever corporation, a major producer of seed-oil-containing foods. Due to their data, they were not able to look at serum levels.
A number of other studies have examined the relationship of serum Ω-6 and T2DM, and found that lower serum Ω-6 is associated with higher rates of T2DM (Saloma et al., 1990; Wu at al., 2017—another Unilever effort). The interpretation is that Ω-6 is protective against T2DM, in contradiction of the historical fact that increased Ω-6 PUFA and decreased saturated fat consumption has accompanied increased rates of T2DM (Lee, et al., 2022).
Inflammation is common in diseases.
Inflammation is common in diseases, if not universal, as it is a beneficial part of the healing process. It’s a major part of ARDS, discussed in the post above, and is also a part of diabetes. The ARDS experience and the Hodge study suggest that inflammation decreases Ω-6 in serum.
But does this happen outside of ARDS?
Myelin, the sheath around the connections between nerve cells, is composed primarily of cholesterol and fats, and demyelination is the damage to the sheath; this is the signature feature of multiple sclerosis (MS). In this study, Stanzani et al. are hoping to determine changes in the fatty acid and cholesterol balance and content in the brain during diseases like MS. This might help in designing therapies which could reduce the progression of the disease, as a number of dietary therapies have been proposed.
“Erythrocyte Plasma Membrane Lipid Composition Mirrors That of Neurons and Glial Cells in Murine Experimental In Vitro and In Vivo Inflammation” (Stanzani et al., 2023) explored the inter-relationship between fatty acid composition of red blood cells (RBC) and neurons and glial cells (another type of brain cell), in hopes that measuring RBC might provide a window into the brain. It’s obviously very easy to measure RBC and very difficult to extract brain cells, so, as the title suggests, this positive finding could be quite useful..
To simulate MS, they use “experimental allergic encephalomyelitis (EAE)”:
“EAE is characterized by intense inflammation and the extended demyelination of the spinal cord, following a defined clinical course that includes an acute and a remission phase.” (Stanzani et al., 2023)
The remission part is key, because they are trying to determine what changes to fats occur during inflammation and remission may be driving the progression of the disease.
This is not, to be clear, an intervention that is attempting to alter the course of the disease, they are trying to establish easily-tracked biomarkers that could be used to monitor the disease:
“…the use of peripheral lipids as “biomarkers” to reflect the lipid composition of brain membranes is an attractive and challenging objective, also in view of the ‘Holy Grail’ in the field—the search for a peripheral, noninvasive and robust biomarker reflecting CNS composition.” (Stanzani et al., 2023)
Red blood cell fats change in response to inflammation
This is somewhat expected; the lipid membranes of cells contain polyunsaturated fats (PUFA) that are used as the basis for various hormones in the body, especially during inflammation. This is the basis for the mechanism of 75% of the drugs sold by weight in the world (Rice 2020); blocking the pathway from cell-membrane PUFA to inflammatory mediators. Ω-6 and -3 PUFAs are the sources for these mediators. They’re REMOVED from the lipid membranes by enzymes such as phospholipases, which make them available to other enzymes such as COX and LOX which ACTIVATE them into inflammatory mediators. So the membranes represent the inverse of the fats involved in the inflammatory process.
And indeed, Stanzani et al., found a decline in the PUFAs in RBC during their experimental inflammation. Moreover, this decline mirrored what was happening in the spines of the experimental animals during inflammation.
What was surprising was that the mediators that are usually understood to be altered in inflammation didn’t show much change. Ω-3 fats declined more than Ω-6 did, and so the ratios tracked (Ω-6/Ω-3, Arachidonic acid (AA)/EPA and AA/DHA, and Omega Balance (Hulbert, 2023)) all reflected this.
Ep. 15: A.J. Hulbert on the Omega Balance—with Dr. Brian Kerley
Introduction We are honorored to be joined by Prof. A. J. Hulbert, noted zoologist and author of Omega Balance: Nutritional Power for a Happier, Healthier Life. “In Omega Balance, noted scientist Anthony J. Hulbert explains how the balance between [Omega-3 and omega-6 fatty acids] in the human food chain has changed over the last half-century and the very…
AA, thought to be a primary source of inflammatory mediators changed less than may have been expected, while the AA precursors linoleic acid (LA) and DGLA (an intermediate between LA and AA) were also both decreased. Phospholipases have different affinities for fats, so it is likely that they were all being pulled out of the cells simultaneously. Unfortunately we can’t tell where they all went from this study.
However we can observe that in an inflammatory state like that induced here, serum levels of PUFA will not be an accurate reflection of intake.
Similarly, levels of saturated and monounsaturated fats (SFA and MUFA) went up. The often-maligned SFA palmitic acid was fairly stable, despite its purported role as pro-inflammatory fat (Lancaster et al., 2018), however the ‘neutral’ stearic acid went more. Stearic can be a precursor to MUFA.
Resolution of inflammation causes more changes.
Now the interesting thing about this model for MS is that it also has a remission phase.
As inflammation subsided, the PUFAs went back up. (See the REM columns in the Fig. 2 graphic above.) SFA went down, and MUFA also went up. One suspects that MUFA went up because some of the slots that had been filled by PUFA in the RBC and spinal membranes were now filled with MUFA, as PUFA is a rarer fat in the body. Those spaces in the phospholipids (called ‘sn-2’) can be filled by either PUFA or MUFA, typically. “…the PUFA level restoration was largely due to increases in Ω-6 linoleic and DGLA levels.”
The diet never changed.
“The observed remodeling process had an important metabolic significance, given that the diet consumed by the mice did not change during the experiment. The experimental animal diet was based on an omega-6/omega-3 precursor ratio (linoleic/alpha-linolenic acid ratio) of 8.8; however, the total omega-6/omega-3 ratio was 5.2 due to the presence of long-chain PUFA, EPA and DHA…. It might, therefore, be expected that omega-6 would have been metabolically balanced by omega-3 under our experimental conditions, as described for the animal diet with the omega-6/omega-3 ratio lower than nine [47], which did not occur.” (Stanzani et al., 2023)
Thus, “The changes that we observed during the three phases were, therefore, a direct consequence of the health status induced by EAE.”
It was purely the result of the inflammatory process.
Conclusion
This demonstrates a couple of important points.
Membrane Ω-6 other than AA are directly involved in the inflammatory process, through being converted to AA through the established pathways, or through other enzymes such as CYP450 to less recognized pathways like to leukotoxin (Jonnalagadda et al., 2021).
Low levels of RBC Ω-6 or -3 fats in an inflammatory state should not, necessarily, be considered symptoms of insufficient intake. As demonstrated in ARDS, supplementation of pro-inflammatory fats in an inflammatory process can prove fatal.
And most importantly, that it’s important to be skeptical of scientists with a bias who promote ideas that contradict the evidence.
Astonishingly:
“The membrane lipid relationship described in our study also represents, to the best of our knowledge, the first description of a peripheral cell (RBC) over the entire course of EAE, from onset to remission…” (Stanzani et al., 2023)
I can’t think of a single study that has examined the flux of fats through an inflammatory episode such as this.
Yet many studies make a claim that’s refuted by this paper, that low levels of PUFA are necessarily causal of inflammatory conditions, even when those conditions exist in a high-PUFA food environment.
References
Goodrich, T. (2020, June 2). Does Consumption of Omega-6 Seed Oils Worsen ARDS and COVID-19? [Blog]. Yelling Stop. https://open.substack.com/pub/tuckergoodrich/p/does-consumption-of-omega-6-seed
Hulbert, A. J. (2023). Omega Balance: Nutritional Power for a Happier, Healthier Life. Johns Hopkins University Press. https://amzn.to/3uC2igY
Jonnalagadda, D., Wan, D., Chun, J., Hammock, B. D., & Kihara, Y. (2021). A Soluble Epoxide Hydrolase Inhibitor, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) Urea, Ameliorates Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences, 22(9), Article 9. https://doi.org/10.3390/ijms22094650
Kuriki, K., Nagaya, T., Tokudome, Y., Imaeda, N., Fujiwara, N., Sato, J., Goto, C., Ikeda, M., Maki, S., Tajima, K., & Tokudome, S. (2003). Plasma Concentrations of (n-3) Highly Unsaturated Fatty Acids Are Good Biomarkers of Relative Dietary Fatty Acid Intakes: A Cross-Sectional Study. The Journal of Nutrition, 133(11), 3643–3650. https://doi.org/10.1093/jn/133.11.3643
Lancaster, G. I., Langley, K. G., Berglund, N. A., Kammoun, H. L., Reibe, S., Estevez, E., Weir, J., Mellett, N. A., Pernes, G., Conway, J. R. W., Lee, M. K. S., Timpson, P., Murphy, A. J., Masters, S. L., Gerondakis, S., Bartonicek, N., Kaczorowski, D. C., Dinger, M. E., Meikle, P. J., … Febbraio, M. A. (2018). Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metabolism, 27(5), 1096-1110.e5. https://doi.org/10.1016/j.cmet.2018.03.014
Quinlan, G. J., Lamb, N. J., Evans, T. W., & Gutteridge, J. M. C. (1996). Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Read Online: Critical Care Medicine | Society of Critical Care Medicine, 24(2), 241–246. https://doi.org/10.1097/00003246-199602000-00010
Rice, M. E. (2020). Bruce D. Hammock: Science Should Be Fun. American Entomologist, 66(1), 14–19. https://doi.org/10.1093/ae/tmaa010
Salomaa, V., Ahola, I., Tuomilehto, J., Aro, A., Pietinen, P., Korhonen, H. J., & Penttilä, I. (1990). Fatty acid composition of serum cholesterol esters in different degrees of glucose intolerance: A population-based study. Metabolism, 39(12), 1285–1291. https://doi.org/10.1016/0026-0495(90)90185-F
Stanzani, A., Sansone, A., Brenna, C., Baldassarro, V. A., Alastra, G., Lorenzini, L., Chatgilialoglu, C., Laface, I., Ferreri, C., Neri, L. M., & Calzà, L. (2023). Erythrocyte Plasma Membrane Lipid Composition Mirrors That of Neurons and Glial Cells in Murine Experimental In Vitro and In Vivo Inflammation. Cells, 12(4), 561. https://doi.org/10.3390/cells12040561
Wu, J. H. Y., Marklund, M., Imamura, F., Tintle, N., Korat, A. V. A., Goede, J. de, Zhou, X., Yang, W.-S., Otto, M. C. de O., Kröger, J., Qureshi, W., Virtanen, J. K., Bassett, J. K., Frazier-Wood, A. C., Lankinen, M., Murphy, R. A., Rajaobelina, K., Gobbo, L. C. D., Forouhi, N. G., … Mozaffarian, D. (2017). Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. The Lancet Diabetes & Endocrinology, 5(12), 965–974. https://doi.org/10.1016/S2213-8587(17)30307-8










Over time I've become suspicious of Omega Quant reports. I've been using them for about 5 years. When I had a MI I had some really wacky numbers, especially AA: EPA 42. High N6 and very low N3. After that I started in earnest with LA out of my life. Reports better toward "optimium " range without fish oil or fish. Finally, my last report was absolutely a stunner......everything in the green. I wonder if the Omega 6:3 "normalized" by smoldering inflammation and O3 bolstered by supplements. Just a thought given that I have plenty of aches and pains. Say it ain't so Joe!