A Landmark Paper on Seed Oils and Obesity— Sigh
tldr; The details of how one performs a study matter a lot. Unfortunately this paper doesn't really clarify things.
A Dream Paper?
I’ve many times referred to (Alvheim et al., 2012) as it was one of the first papers to rigorously look at linoleic acid, the primary fat in seed oils, and obesity, and as the results have been replicated numerous times—discussed in (Goodrich 2021) and (Goodrich & Kerley, 2023).
This paper looked at varying levels of Ω-3 and Ω-6 fats, attempting to model the changes that had happened in the American food supply over the 20th century, and to determine if those changes were:
Related to the emergence of obesity.
Could be tied to changes in Ω-6-derived hormones.
They confirmed both hypotheses: Mice given a modern diet developed obesity, while mice given a more traditional diet did not. And the differences were correlated with increased levels of hormones known as endocannabinoids, which are involved in the control of appetite and energy balance. Additionally, they found that increased levels of Ω-3 fats were protective against obesity.
However, that paper was performed on mice. Mice are good models, but they’re mice, not people, and what we really care about are people.

A couple of similar studies have been performed on people, and suggest that this mechanism should hold in people, as one would expect it to. Note the declining intake of Ω-6 fats is accompanied by declining weight.
But ideally what you would want to see is the same or similar experiment being done in humans.
Practically speaking, that can’t happen. Alvheim took pregnant mice and raised their male offspring until the end of the study, when they were dissected. I’ll spare you that picture.
And given the fact that all Americans at this point ARE those mice, that is, we’ve all been raised on a high-Ω-6 diet since birth, you couldn’t just take a bunch of Americans, put them in cages, and feed them the obesogenic levels of Ω-6. That’s what they’ve been eating. It’s too confounded.
So, the same authors (hallelujah!) did the reasonable thing to do in humans in (Courville et al., 2023). They took a bunch of overweight Americans (all women, for some reason they don’t discuss) and put them on three experimental diets, based on the previous work in Alvheim.
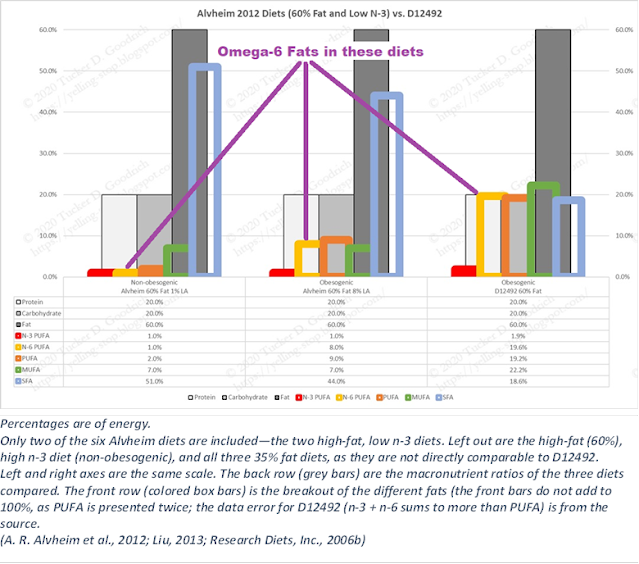
So here are the various diets from 2012 (only looking at the 35% fat diets) and the 2023 diets (which are all 30% fat). The big difference is that in 2012 they had a H6H3 diet, that was 8% Ω-6 and 2% Ω-3, with added DHA and EPA of 1%; whereas in 2023 they have a low Ω-6 and ‘high’ Ω-3 diet. (All these figures are as percentages of energy consumed, for ease of comparison.)

You will also note that the high Ω-3 diet was 2% in 2012, whereas in the human experiment of 2023 it is only 1%, which is the same as the baseline diet. So they effectively don’t have a high Ω-3 diet. Both human low Ω-3 diets are 0.5%, also half of the 2012 diets.
It’s simpler to look at the Omega Balance (Hulbert, 2023) of the various diets, of course.
The baseline Ω-6 and Ω-3 intakes for these women in 2023 are spot-on the estimate that was used as the basis for the 2012 paper: 8% Ω-6 and 1% Ω-3. According to that, they should be over-weight, and they are.
They Really Ate That Little?
I’m also surprised by these calorie figures (only provided for the 2023 human diets). Their baseline consumption was 1994 Calories for the entire group, assessed as follows:
“Women kept food records for 7 days prior to randomization to capture pre-study dietary intake. At the second screening visit, food records were reviewed with nutrition staff for completeness and portion sizes were verified using three-dimensional food models and other visual aids such as measuring cups.”
That seems low to me. Without going too into the weeds here, there are lots of issues with food reporting, as overweight people especially tend to under-report what they eat.
“Women are more likely to under-report than men, and under-reporting is more common among overweight and obese individuals.”
“…it is necessary to distinguish between under-reporting and genuine under-eating for the duration of data collection.” (Macdiarmid & Blundell, 1998)
Despite this high risk of under-reporting, or accurate reporting and under-eating during the reporting phase, Courville et al. take their subjects’ reports at face value, and set the calorie levels for the experimental diets at this ~2000 Calorie level.
This “Estimated Energy Requirement” calculator also suggests 2000 calories is too low for these women to maintain weight:

As you can see, however, all the groups eat above their baseline level. More about this later.
Changes in Weight
This is a short-term study, as such studies in people tend to be. It’s also high-quality, as the food was mostly provided by the study to the subjects. There was some cheating, of course, but they tried to track that.
They had two phases of the diet:
“During the first 4 weeks, participants were on an iso-caloric “weight maintenance” phase, where they were fed their estimated energy needs (estimated resting energy expenditure (Mifflin et al., 1990) multiplied by an activity factor representative of their habitual activity (Otten et al., 2006) and the energy provided was adjusted in order to keep their daily body weight ± 2 kg of their fasting body weight measured on the first day of the diet.”
2 kilograms is 4.4 pounds, so that’s a normal weight variation. They don’t report whether subjects needed extra food or ate less food during this phase, just the weight change.
“During the following 8 week “ad libitum” phase (from 5 to 12 weeks) to assess the impact of the diets on ad libitum daily intake, participants received the same diets but were provided with 500 kcals less in their daily meals, however an additional 500 kcals of snacks were provided that matched the overall diet composition to which they were assigned. Participants were instructed to consume the snacks ad libitum according to their perceived hunger level while consuming all meals in their entirety.”
I don’t see what the point of those instruction are, except it probably made it easier for the researchers to track it. I don’t like nit-picky instructions in diets like this, as they are affecting the thing they are trying to measure. They should just let people eat, and figure out a better way to to track consumption.
“During the first 4 weeks, women’s body weight remained stable (H6L3, -1.7 ± 1.8; L6L3, -1.1 ± 1.3, and L6H3, -0.9 ± 1.4, p = 0.186).”
You’ll note that correlates with the reported over-eating of 103%, 104%, and 107% of baseline, respectively.
“From weeks 5 to 12, women on each diet lost a similar amount of weight (H6L3, -2.4 ± 3.1; L6L3, -2.4 ± 2.2 and L6H3, -3.2 ± 2.7, p = 0.574).”
So, as expected, the latter two ‘intervention’ groups lost weight. However, so did the ‘control’ group.
You Need a Real Control
This study doesn’t have a real control group. The H6L3 group was eating a diet that approximated what they were eating before, supposedly, based on the reported pre-study consumption. Yet it was the provided diet, not what they usually ate, and they lost about the same amount of weight as the two other groups.
This suggests that their diet during the study was different in some way from their previous diet.
This drives me nuts about studies like this. How difficult is it to have one group where they measure all the same stuff, then send them home for the duration of the study with no dietary instructions at all?
That would be a real control.
I’d wager all three groups were eating less than their usual intake, and this is what drove the weight loss; it’s due to -300 Calories per day, not to any hormonal effect. Calorie restriction alone induces hormonal changes, and fasting specifically alters the levels of the measured hormones.
“Thus, under physiological conditions when circulating leptin levels are low, such as during fasting or the inter-meal interval, endocannabinoid levels may rise and cause alterations in neurotransmission that promote feeding.” (Lau et al., 2017)
Courville et al., not surprisingly, don’t mention anything about weight loss in the abstract.
About Those Hormones…
So the expectation was that reducing Ω-6, and especially increasing Ω-3, would lower the levels of the hormones that control appetite and energy balance.
That’s not what happened, either.

Both of these hormones are made from the Ω-6 fat arachidonic acid (AA), which is made from linoleic acid, the primary fat in seed oils. Levels of AA also didn’t change. This is odd, as these same authors published a study in 2021 with a similar dietary change that did lower AA.
“Both interventions also significantly decreased n-6 arachidonic acid and its elongation product docosatetraenoic acid in erythrocytes compared with the control diet.” (Ramsden et al., 2021)
However, Courville was conducted from 2011-2018, while Ramsden was designed and conducted later, despite being published first. Ramsden was less successful than Courville in lowering LA, but nevertheless seemed to succeed better in the regard of lowering AA.
One difference between the two studies is that in (Ramsden et al., 2021):
“All three diets were designed to be eucaloric (not promote weight loss).”
However they don’t report calories consumed, either in the base diet or that may have been provided to avoid weight loss. So maybe the lack of decline in hormones was due to caloric restriction, as suggested above? We can’t tell from this.
Another problem is that the hormonal changes that affect weight loss might not occur in serum. AA and the associated hormones are very bio-active, and are tightly controlled. As discussed in this post:
Does Linoleic Acid Induce Obesity? Part 1
[P.S. Part 2: Roux-en-Y Gastric Bypass and 4-Hydroxynonenal.] Introduction OK folks, into the wayback machine.Thanks for reading Tucker Goodrich: yelling Stop! Subscribe for free to receive new posts and support my work. In 2010 I stopped eating seed oils, and saw a chronic inflammatory bowel disease resolve in days:
The changes that matter to appetite happen in the gut, not in serum. They aren’t really able to measure that in free-living humans, however.
Another confounding factor is that these hormones seem to lower metabolic rate, at least blocking them through drugs or surgery increases metabolic rate, as discussed in that post. Calorie restriction also lowers metabolic rate. Is it because it increases the endocannabinoids? It’s likely. I keep coming back to under-consumption of calories being a basic problem here.
Conclusion
So it’s a landmark study, that didn’t produce the expected result.
Yes, the intervention group lost weight, but it wasn’t associated with the changes in hormones. And the control group also lost weight, for reasons that aren’t entirely clear.
“This study had several important limitations. Since LA is enriched in the adipose tissue of United States adults (Guyenet & Carlson, 2015), the half-life of LA in adipose tissue is believed to be ~2 years in humans (Dayton et al., 1968), therefore a much longer study may be needed to observe the full effects of LA lowering on circulating LA and ARA…. The eucaloric feeding period of the study was short and the inclusion of an ad libitum portion of the study resulted in weight loss in many women within all three diet groups which could have influenced changes in FA and ECB during the last 8 weeks of the study. Furthermore, we only have static measures of plasma FA and ECB and we were unable to measure FA stored or being released from adipose and other tissues. Future studies are therefore needed to understand the effects of a diet on the flux of FA and their ECB derivatives in humans.” (Courville et al., 2023)
Science is tough. I’d love to hear the authors’ thoughts one what went on here, beyond what they reported in the paper.
(Thanks to Bill Lagakos for pointing this paper out to me.)
References
Alvheim, A. R., Malde, M. K., Osei‐Hyiaman, D., Hong, Y. H., Pawlosky, R. J., Madsen, L., Kristiansen, K., Frøyland, L., & Hibbeln, J. R. (2012). Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity, 20(10), 1984–1994. https://doi.org/10.1038/oby.2012.38
Courville, A. B., Majchrzak-Hong, S., Yang, S., Turner, S., Wilhite, B., Ness Shipley, K., Horneffer, Y., Domenichiello, A. F., Schwandt, M., Cutler, R. G., Chen, K. Y., Hibbeln, J. R., & Ramsden, C. E. (2023). Dietary Linoleic Acid Lowering Alone Does Not Lower Arachidonic Acid or Endocannabinoids Among Women with Overweight and Obesity: A Randomized, Controlled Trial. Lipids, 58(6), 271–284. https://doi.org/10.1002/lipd.12382
Goodrich, T. D. (2021, November 18). Does Linoleic Acid Induce Obesity? Part 1 [Blog]. Yelling Stop. https://tuckergoodrich.substack.com/p/does-linoleic-acid-induce-obesity
Goodrich, T., & Kerley, B. (2023, October 26). Ep. 10: Dalton Graham: How to Induce Fatty Liver—With Dr. Brian Kerley [Substack newsletter]. Tucker Goodrich: Yelling Stop. https://tuckergoodrich.substack.com/p/ep-10-dalton-graham-how-to-induce
Hulbert, A. J. (2023). Omega Balance: Nutritional Power for a Happier, Healthier Life. Johns Hopkins University Press. https://amzn.to/3uC2igY
Lau, B. K., Cota, D., Cristino, L., & Borgland, S. L. (2017). Endocannabinoid Modulation of Homeostatic and Non-Homeostatic Feeding Circuits. Neuropharmacology, 124, 38–51. https://doi.org/10.1016/j.neuropharm.2017.05.033
Macdiarmid, J., & Blundell, J. (1998). Assessing Dietary Intake: Who, What and Why of Under-Reporting. Nutrition Research Reviews, 11(2), 231–253. https://doi.org/10.1079/NRR19980017
Ramsden, C. E., Zamora, D., Faurot, K. R., MacIntosh, B., Horowitz, M., Keyes, G. S., Yuan, Z.-X., Miller, V., Lynch, C., Honvoh, G., Park, J., Levy, R., Domenichiello, A. F., Johnston, A., Majchrzak-Hong, S., Hibbeln, J. R., Barrow, D. A., Loewke, J., Davis, J. M., … Mann, J. D. (2021). Dietary Alteration of N-3 and N-6 Fatty Acids for Headache Reduction in Adults with Migraine: Randomized Controlled Trial. BMJ, 374, n1448. https://doi.org/10.1136/bmj.n1448







There is an obsession with RCTs even if there are confounding factors, which seems daft to me. I guess it's a publication to go on the CV. This is something that could most easily be done with people in long term care where food ingredients are fully under the control of the institution. Apart from Ω-3 and Ω-6 levels everything else can be the same for two RC groups.