Thoughts on 'Of Rats and Sidney Diet Heart...', Alan Flanagan's Post Defending Seed Oils
This is from a couple of years ago, but if you aren't familiar with the hijinks those attempting to defend the nutritional malpractice will attempt...
Apologies to Stan Efferding, who asked me last year to look at this blog post. I really don’t like doing this kind of post, especially when it’s a soft target, but as it keeps coming up, I guess I have no choice.
So here we go.
Introduction

The blog post in question is titled “Of Rats and Sydney Diet-Heart: Drawing a Line Under Polyunsaturated Pseudoscience” (Flanagan, 2020b), the author is Alan Flanagan, who “…has an MSc in Nutritional Medicine, is currently pursuing his PhD, and a [sic] former practicing Lawyer (Barrister) from Dublin, Ireland.” He states, “The core values behind Alinea Nutrition are critical thinking that is clear, objective, rational, free from dogma, and informed by evidence” (Flanagan, 2020a). Alinea is his website’s name.
He commences his post:
“To say that hysteria about the health effects of polyunsaturated fats [PUFA] has reached fever pitch may be an understatement.
“It can be interesting watch [sic] a trend of thought gain traction when that train of thought is inconsistent with a substantial total body of evidence, from multiple converging lines of inquiry.
“It speaks to something at play beyond scientific discourse, and frankly much of the “debate” regarding PUFA is steeped in a mix of narratives, from fantasies about our evolutionary past to conspiracy theories about governments, scientists, the food industry, and ‘BigPharma’.
“I have a few thoughts as to why the PUFA-hysteria appears to have exponentially increased in recent years. It’s likely not an exhaustive list, but these are some of the more common narratives I’ve observed….” (All quotes from (Flanagan, 2020b) are bolded and not further footnoted.)
Well, I’ve been told I’m in a good part responsible for the “PUFA-hysteria”, so I guess I have an interest in defending it.

He starts his argument with a straw-man. The image which opens his blog post is of rape (the flower), rapeseed (the seed pods), and rapeseed oil (aka Canola oil). There are two notable types of PUFA fats, omega-6 (n-6 or Ω-6) and omega-3 (n-3 or Ω-3). Ω-6 is that which is contained in rapeseed oil (basically), and Ω-3 is that which we get from pastured or wild meats and fish (again, basically).
Both Ω-3 and Ω-6 fats have concerns about them, but to lump them together like this is disingenuous, as Ω-3 is regarded universally as necessary for human health. There are a small group of people who are concerned about the effects of excess Ω-3 fats in the human diet. (These are those who read the writings of Ray Peat (Peat, 2007), who, unlike Flanagan, has a PhD in biology with a focus on physiology (Peat, 2006), the relevant area for this discussion.) Most people, even in nutrition, have never heard of Ray Peat or his followers, and to claim they’re causing a “hysteria” against Ω-3 PUFAs is ridiculous.
Since Flanagan never provides any evidence that anyone is advocating against Ω-3 consumption (he doesn’t mention Peat or his followers) one can only conclude that he’s opening his argument in bad faith, by tying an unsupportable (and hence, unsupported) argument with an argument he is attempting to discredit. (This is the Association Fallacy, so perhaps he’s going to work through the entire list of fallacies? He’s hit two already, and we’re not yet out of the introduction!)
So what he’s actually complaining about (assuming he’s not completely unaware of this “debate”) is Ω-6 PUFA.
“The surge in popularity of ‘ancestral’ viewpoints of health…”
“The populist, modern incarnation of the ‘Paleo’ diet seems divorced from the evidence. Pay attention to any ‘guru’ in this area, and you’ll inevitably hear an emphasis on saturated animal fats, as if this nutrient formed a backbone of the ancestral diet…. Saturated fat are estimated to have provided about 6% of the average total energy intake for humans in the Paleolithic period, reflecting the fat composition of wild game meat in free-living African mammals.”
I’m a little unclear as to what “populist, modern” Paleo diet he’s referring to. Perhaps it’s Loren Cordain’s book, The Paleo Diet (Cordain, 2010)? That would be odd, as the first link he provides to refute the alleged position of the Paleo diet on saturated fats (copied above) is to “Humans, lipids and evolution” (Eaton, 1992). Odd indeed, as Eaton and Cordain have co-authored many papers and letters together, and in the introduction to The Paleo Diet, Cordain says:
“I am particularly indebted to my friend and colleague S. Boyd Eaton for enlightening me with his seminal New England Journal of Medicine article ‘Paleolithic Nutrition’…” [referring to (Eaton & Konner, 1985)]
So to refute the alleged pro-saturated fat premise of the Paleo diet, Flanagan cites: the founder of the Paleo diet? This seems like another strawman argument, but more likely we’ll have to just assign it to ignorance of the topic being criticized. Sloppy ignorance, at that.
This letter from Eaton and Cordain might as well have been addressed to Flanagan for his misrepresentation of the Paleo diet:
“Nowhere in our article did we recommend that people should eat high-[saturated] fat, domesticated livestock. Our take-home messages were that hunter-gatherer diets were higher in protein and lower in carbohydrate than are current Western diets or dietary guidelines and that this macronutrient balance may provide insight into potentially therapeutic diets. If any implication were to be inferred, it would be that dietary fat should emulate fat sources found in game meat and organs (high in n3 fats, low in n6 fats, and high in monounsaturated fats).” (Cordain et al., 2000)
Not only is an updated version of the anti-saturated fat data from (Eaton, 1992) included as Appendix B of The Paleo Diet (Cordain,2010, p. 215), but one of the core principles of the Paleo diet is, “No dairy products” (Cordain, 2010, p. 20), which are the primary source of saturated fat in the modern diet. Flanagan doesn’t mention this, of course, in his lame attempt to smear the Paleo diet with the taint of saturated fats. Nor does Flanagan mention how saturated fat has largely been vindicated, in multiple large papers (Bhavadharini, 2020; Hooper, 2020; Micha, 2010; Siri-Tarino, 2010):
“We found little or no effect of reducing saturated fat on all-cause mortality (RR 0.96; 95% CI 0.90 to 1.03; 11 trials, 55,858 participants) or cardiovascular mortality (RR 0.95; 95% CI 0.80 to 1.12, 10 trials, 53,421 participants), both with GRADE moderate-quality evidence.” (Hooper, 2020)
Instead, Flanagan goes back to his straw-man, citing the many, undisputed benefits of Ω-3 fats in health and evolution. He still provides no evidence that anyone disagrees with the inclusion of these fats in the diet.
Despite titling this section “The surge in popularity of ‘ancestral’ viewpoints of health, arguments based on assumptions about our evolutionary diet…”, he doesn’t explain what might underly that “surge”, and he implies there must be something wrong with such arguments, although he again doesn’t provide any evidence for it (This is both the Failure to State and the Argument by Dismissal; I’m not going to keep track of any further fallacies he perpetuates. I don’t have the time!). This might come as news to another source he fails to mention. The Story of the Human Body: Evolution, Health, and Disease is the title of (Lieberman, 2013). In my last comparison to Flanagan’s academic credentials, Lieberman, like Cordain (Eaton is an MD) is a PhD. At the time of writing that book, he was also the Chair of the Department of Human Evolutionary Biology at Harvard University, and a world leader in his field. If the Paleo diet hadn’t existed before Lieberman’s book was written, he would have brought it to life. It’s a masterpiece of looking at the evidence for a mismatch between our modern diets and our evolutionary heritage.
Flanagan should read it.
In short, we’ve had a surge in the popularity of ancestral viewpoints of health because scientists have been making the argument, with increasing success, that our divergence from our ancestral way of living is at the root of our pandemic of ill health. That’s what science should be doing: showing us a path toward solutions, not casting baseless, ignorant aspersions.
“Ability to find rodent studies to corroborate beliefs about PUFA.”
“It’s odd that such studies in our rodent friends are used to support theoretical arguments of human evolution harking back to the Palaeolithic [sic] period.”
It’s remarkable that Flanagan got through to a Masters of Science without understanding why this is valid, or why it’s the standard approach in science. Prof. Lieberman, for instance, did a seminal experiment on meat and chewing in humans (Zink, 2016), but another, earlier experiment had to look at the effects of chewing in an animal model:
“Hyraxes raised on cooked food had significantly less growth (approximately 10%) in the ventral (inferior) and posterior portions of the face, where strains are highest, resembling many of the differences evident between humans raised on highly processed versus less processed diets. The results support the hypothesis that food processing techniques have led to decreased facial growth in the mandibular and maxillary arches in recent human populations.” (D. E. Lieberman et al., 2004)
Obviously, such an experiment could not be done on human children. Somehow, despite using these “odd” methods, he nevertheless became Chair of such a prestigious department.
Moreover, the field of toxicology overwhelmingly uses animal models, it’s the standard approach, not “odd” (National Research Council, 2000). Mustard oil, for instance, is banned from sale for human consumption in the United States solely on the results of animal experiments:
“Expressed mustard oil is not permitted for use as a vegetable oil. It may contain 20 to 40% erucic acid, which has been shown to cause nutritional deficiencies and cardiac lesions in test animals.” (U.S. Food & Drug Administration, 2016)
Flanagan continues:
“To say nothing of their lack of translational evidence in humans. Human outcomes > rodent mechanisms. That is how we evaluate strength of evidence.”
He provides no supporting evidence for this inaccurate claim here, however further on he does discuss the translational evidence in humans. We will discuss that below.
“Play on ‘BigFood-BigPharma-wants-to-make-you-sick’ emotive narratives.”
He makes some claims here, but again provides no evidence.
“This also tends to come hand-in-hand with the “it all started with Ancel Keys” diatribe, regurgitated by automatons who haven’t even read his research, or even of the wider diet-heart literature, from that period. I’ve written an extensive article on the early metabolic ward studies from that period, and the various research groups involved, which you can read elsewhere on site.”
The link doesn’t go to an article, so it’s not possible to determine if that provides any evidence. He does bring this up again later on, so we will discuss then. Since we’re no longer keeping track of his logical fallacies, we’ll just note that there are more here.
“It’s easy to build a pseudoscientific case against PUFA.”
After thin gruel so far, we’re starting to get to the meat of his argument. Note, it’s also easy to build a pseudoscientific case for PUFA. Let’s see how he does.
“The gravamen of the argument for PUFA being potentially deleterious for human health centres on two processes: inflammation and oxidation, specifically, lipid peroxidation…. Additionally, that the omega-6 linoleic acid acts as a precursor to pro-inflammatory eicosanoids, and therefore that PUFA cause adverse effects through inflammation.”
Ignoring that he’s again conflating that Ω-6 and Ω-3 cause “adverse effects”, this is indeed the core of the argument.
“The exact same rhetorical hack is deployed with much of the claims regarding PUFA. “Well you see they’ve a double-carbon bond at the omega-6 position” as if this provides evidence of a conclusion of disease risk in humans, or “well you see linoleic acid is a precursor to arachidonic acid, and that is pro-inflammatory”, as if this provides evidence of an actual inflammatory effect. What they are not telling you, or asking, is the answer to the fundamental question: is this physiologically relevant in humans? Does this putative mechanism translate to an in vivo effect?”
Again, no evidence that anyone is actually doing this, but that’s pretty obviously his “rhetorical hack”.
Inflammation
Finally, we start to get to some actual science, and some claims we can evaluate beyond “no evidence provided!”:
“Let’s start with inflammation. The putative mechanism with regard to inflammation is that the omega-6 linoleic acid [LA] acts as a precursor to arachidonic acid [AA]; AA acts a substrate to form eicosanoids, and the eicosanoids AA is used to form may be pro-inflammatory. Ergo, as the logic goes, increasing PUFA – particularly LA – increases inflammation.”
Well, that’s not the most logical starting point, as one generally doesn’t start in the middle of the story, and of course he doesn’t quote anyone actually saying this. He continues:
“Except there is no evidence that either increasing or decreasing LA levels alters levels of AA in humans. A review of 36 human intervention studies [his link to (Rett & Whelan, 2011)] highlighted that neither increasing LA levels by up to 551%, or decreasing LA levels by 90%, altered concentrations of AA in plasma, serum, or red blood cells [erythrocytes], despite increasing LA intake resulting in increased membrane phospholipid LA content. Putative mechanism does not = biological effect. The following illustration helps to illustrate why:”

See diagram above (provenance unknown, he does not list a reference, but as it does not represent what he describes it as representing—see D6D—it’s unlikely that he created it).
This is, believe it or not, a necessarily drastically over-simplified version of metabolism and inflammatory pathways in which n-6 is involved! It leaves out quite a few well-recognized inflammatory pathways, however.
As that image makes clear, there are many different pathways, not just the one Flanagan provides the studies for. (Yes, this begins to look like another strawman, but we’re not keeping track!)
So let’s start by looking backwards, from the most well-recognized and effective marker/mediator of inflammation, C-reactive protein (CRP) (Watson et al., 2019)—which is not in the image above. CRP isn’t just a marker, it’s an active player in the body’s immune system:
“...it can be said that CRP possesses the functionality of a host defense molecule against not only atherosclerosis but against all diseases caused by proteins when proteins behave like a pathogen or a toxic molecule, in a life cycle that begins as free CRP in circulation and ends in ligand-bound mCRP at sites of inflammation...” (Singh & Agrawal, 2019)
Oxidized LDL (oxLDL) is such a toxic molecule, and indeed one of the roles of CRP is to bind to oxLDL and help remove it:
"...CRP also binds to the PC moiety of oxidized phosphatidylcholine [PtC] present in OxLDL and apoptotic cells, where CRP triggers the early steps of the classical complement pathway..."
“In addition, CRP only bound to unsaturated PtC in proportion to their degree of oxidation and unsaturation (Fig. 2A) and did not bind to the saturated PtC even if exposed to the same oxidizing conditions (Fig. 2A)." (Chang et al., 2002)
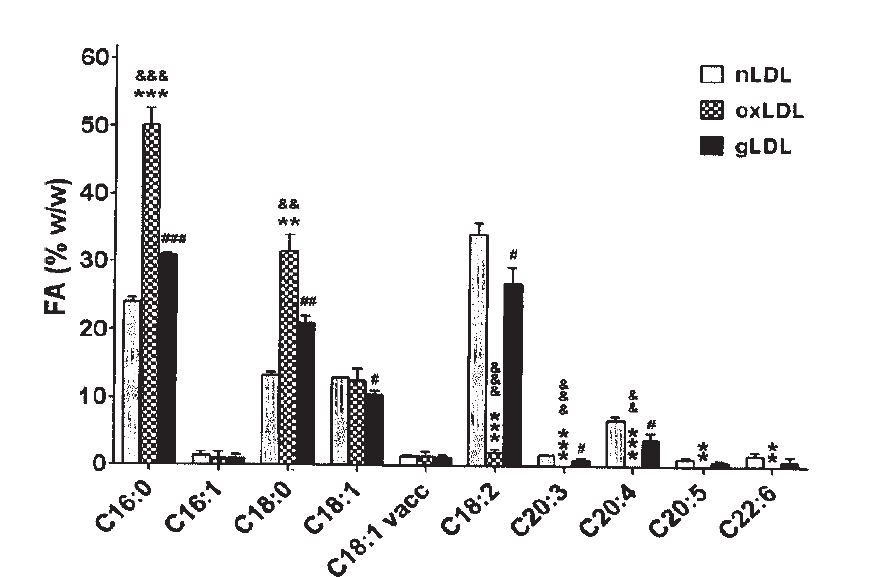
We’ve known since the 1980s that what is oxidizing in LDL is Ω-6 fats, (Deleanu et al., 2016) and that:
“The nature of the substrate for lipid peroxidation, mainly the polyunsaturated fatty acids in lipid esters and cholesterol, is a dominant influence in determining susceptibility. As noted by Esterbauer et al. (52), there is a vast excess of polyunsaturated fatty acids in LDL, in relationship to the content of natural, endogenous antioxidants. The importance of the fatty acid composition was impressively demonstrated by our recent studies of rabbits fed a diet high in linoleic acid (18:2) or in oleic acid (18:1) for a period of 10 wk. LDL isolated from the animals on oleic acid-rich diet were greatly enriched in oleate and low in linoleate. This LDL was remarkably resistant to oxidative modification, measured either by direct parameters of lipid peroxidation (i.e., TBARS and conjugated dienes) or by the indirect criterion of uptake by macrophages (53)….
“In a recent study, human volunteers were fed a similar oleic acid-rich diet. When their
LDL was tested for susceptibility to oxidative modification, it was reduced albeit to a lesser degree than that noted in the rabbit studies (54). These studies demonstrate the feasibility of dietary modification of LDL fatty acid content in order to reduce its susceptibility to modification.” (Witztum, 1991)
Multiple studies have confirmed that dietary manipulation can alter the fatty-acid composition of LDL, and hence its susceptibility to oxidation (Abbey, 1993; Hargrove, 2001; Parthasarathy, 1990; Reaven,1994; Spiteller, 2000).
Thus:
“In conclusion, our data show that Ox-LDL and hs-CRP levels correlate positively in ACS [acute coronary syndrome] patients, supporting the hypothesis that Ox-LDL and CRP may play a direct role in promoting the inflammatory component of atherosclerosis in these individuals.” (Zhang et al., 2012)

Notably, while CRP is a reaction to inflammation, oxLDL is a cause of inflammation, due to its oxidized, dietarily induced Ω-6 fats (Hao, 2015; Kennedy, 2011; Norris, 2011; Que, 2018; Shapira, 2008; Stiekema, 2019; van der Valk, 2016; Witztum, 2002) and ad nauseum, if you are interested.
So where does this get us as to diet and inflammation? (Bemelmans, 2004) looked at the question directly:
“Because of the lower CRP level, the present results suggest that a six-fold increased ALA intake may have anti-inflammatory effects, when investigated against an LA-rich background diet.”
While (Su, 2017) performed a systematic review and meta-analysis of RCTs in humans and found:
“However, in subjects with greater increase in LA intake, LA tends to increase the blood concentration of CRP.”
There are a number of caveats and conditions which are not discussed here, and a discussion of the scope of the inflammation induced via oxLDL is outside the need for a simple proof that it happens, QED.
So let’s go back to Flanagan. After introducing his little diagram above and his supporting studies, he states:
“This is where the buck just stops for the inflammation argument. There is no evidence that the putative mechanism is operative, nor that there is an inflammatory effect of dietary LA in actual Homo Sapiens.”
Well. Obviously that’s wrong.
Let’s discuss another pathway, demonstration of which is a little less involved than the above, although crucial, path from dietary LA to CRP. This pathway, unlike the rather more obvious one above, is in his diagram.
“Does inflammation have a role in migraine?”
“Migraine is a prevalent disorder, affecting 15.1% of the world’s population…. We propose that the increase in migraine frequency leading to chronic migraine involves neurogenic neuroinflammation, possibly entailing increased expression of cytokines via activation of protein kinases in neurons and glial cells of the trigeminovascular system.” (Edvinsson, 2019)
This hypothesis was tested, in an animal model of course (we will get to why an animal model) in 2020:
“In preclinical models, HODEs, EpOMEs and DiHOMEs have been observed to participate in numerous (patho)physiological processes during inflammatory pain including mechanical and thermal hyperalgesia [excess pain]… We demonstrate two 11-hydroxy-epoxides increased proportions of responsive TNs [trigeminal neurons] in a concentration-dependent fashion, similar to PGE2. Further investigation revealed that exposure produced Ca2+ responses with high potency, at μM range, comparable to well-known pain mediators, LA and 9-HODE (Patwardhan et al., 2010, 2009).” (Doolen et al., 2020)
Here’s Flanagan’s diagram again, this time with the emphasized terms above circled. (I also highlighted soluble epoxy hydrolase (sEH), as this will be mentioned later):
Incidentally, LA is a “well-known pain mediator”? Hmm…
Now, they’re using “our rodent friends” here because they already demonstrated that reducing dietary linoleic acid in humans (and increasing Ω-3) reduces headache/migraine pain. In science, when one notices an effect in humans, one attempts to find out via an animal model what the mechanism is (Flanagan would probably still find this “odd”):
“In this randomized trial, the combination of increasing dietary n-3 fatty acids with concurrent reduction in n-6 LA (the H3-L6 intervention) produced statistically significant, clinically relevant improvements in headache hours per day, severe headache days, and headache-related quality of life compared to baseline, and compared to the n-6-lowering (L6) intervention. Prior to the intervention, this chronic headache population averaged 23 headache days per month and 10 headache hours per day, despite using an average of 6 different headache-related medications per subject.” (Ramsden, 2013b)
Now, before one jumps to the conclusion that it was the Ω-3 fats that had the effect and not the Ω-6 lowering, let’s look at another study, (Pradalier et al., 2001). The title will suffice, I think: “Failure of omega-3 polyunsaturated fatty acids in prevention of migraine: a double-blind study versus placebo.”
(While it’s outside the scope of this discussion, it’s important to note that the Ω-3 and Ω-6 fats interact, via some of the pathways above, and that increasing Ω-3 can replace Ω-6 in tissue, provided you also lower Ω-6. That may explain these results.)
“We hypothesized that hyperactive metabolism of n-6 linoleic (n-6 LA) and arachidonic (n-6 AA) acids, and insufficient metabolism of n-3 eicosapentaenoic (n-3 EPA) and docosahexaenoic (n-3 DHA) acids, contribute to headache pathogenesis."
The success of (Ramsden, 2013b) has led to a further, much larger, test detailed in (Mann et al., 2018), which is ongoing.
One of the really interesting findings in the study was:
“As expected, the L6 intervention reduced erythrocyte n-6 LA and a number of its pronociceptive [pro-pain] derivatives compared to baseline. Unexpectedly, the L6 intervention also reduced a number of pronociceptive HETEs compared to baseline, despite no change in their precursor n-6 AA in erythrocytes.” (Ramsden, 2013b)
So what this tells us is that Flanagan’s argument, that since AA didn’t change in blood, there was no effect possible on inflammation, was wrong.
“Although biochemical effects were less pronounced compared to the H3-L6 group, this L6 intervention did significantly alter erythrocyte fatty acids and their bioactive derivatives in a manner that we hypothesized would reduce pain.” (Ramsden, 2013b)
AA quantity seems to be tightly regulated in serum. Those studies were correct. But there are many other studies showing that the downstream metabolites of AA, like these HETEs, are still affected by dietary LA. This has been demonstrated in many other conditions with inflammatory components, which are outside the scope of this discussion.
I highlighted sEH in Flanagan’s diagram, because it was discovered by Bruce D. Hammock (Kodani, 2015), who recently said:
“Seventy-five percent, by weight, of the drugs sold in the world work on a single pathway called the arachidonic cascade, where arachidonic acid is converted by cyclooxygenase into prostaglandins, which are strongly pro-inflammatory.” (Rice, 2020)
That’s the pathway detailed in Flanagan’s diagram. Overstimulation of that pathway arguably, is our biggest health problem. Aspirin, for instance, prescribed for cardiovascular disease prevention, works on that pathway. Obviously, one of the most common uses for drugs like aspirin is headache.
Hammock further commented, in a recent podcast:
"So we've got... a very fine-tuned biochemical system and we've sort of thrown the monkey wrench into it by eating too much of a good thing with [linoleic acid]." (Gornoski, 2021)
What was it Flanagan said again?
“This is where the buck just stops for the inflammation argument. There is no evidence that the putative mechanism is operative, nor that there is an inflammatory effect of dietary LA in actual Homo Sapiens.”
All this evidence was available when he wrote that. One can only conclude he’s completely unaware of the evidence on this subject. Spreading misinformation about an important health topic like this is not helpful and may prevent many people from undertaking an intervention that could provide significant benefits, as in these migraine sufferers.
Lipid Peroxidation
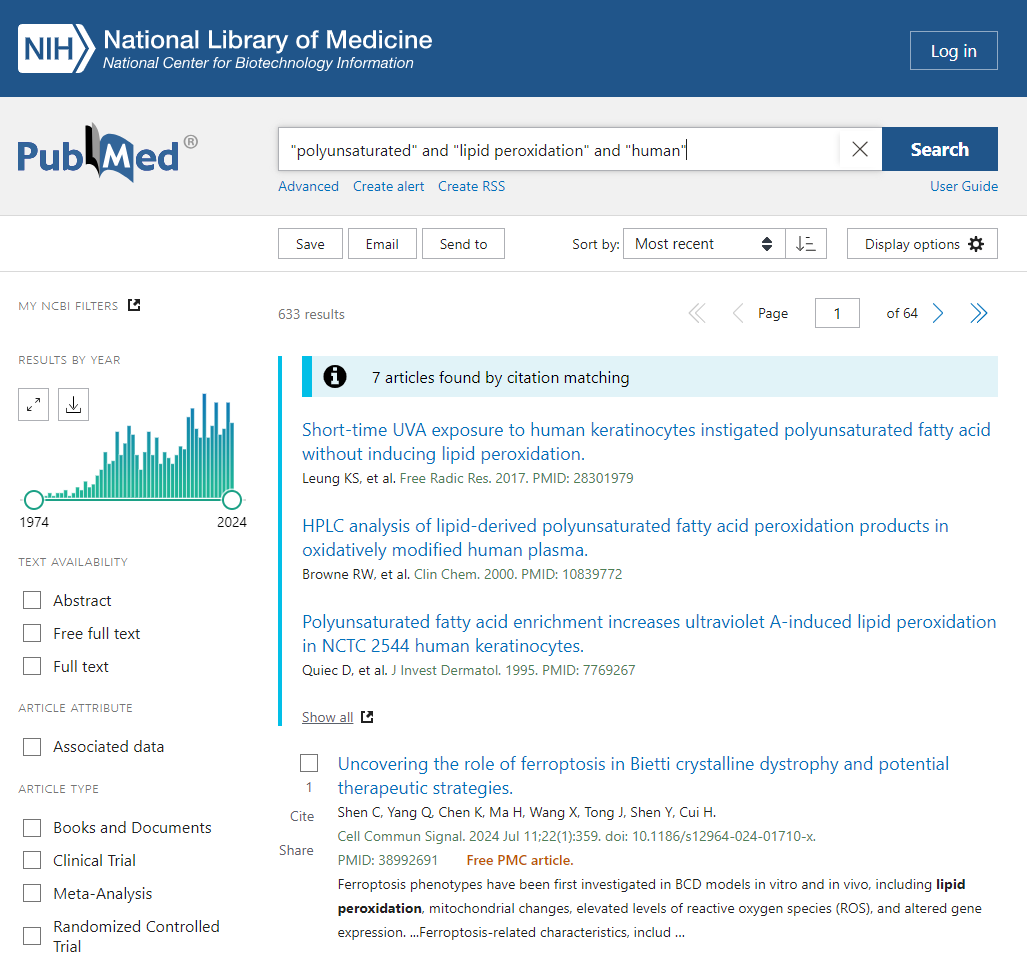
“So, what of lipid peroxidation? A search of the terms “polyunsaturated” and “lipid peroxidation” and “human” in PubMed reveals a small number of trials that have directly tested the effects of PUFA on various markers or surrogate markers of lipid peroxidation in our very own species.”
We already covered this topic above, with oxLDL, which is the product of lipid peroxidation. I’ll simply cite a recent paper that builds upon some of the work of Witztum and Steinberg:
“In conclusion, our findings provide insights that the predictive value of “LDL-C” is potentiated by its Lp(a)-C content.” (Willeit et al., 2020)
What that paper suggests is that the majority of the risk of CVD is dependent on Lp(a), a type of oxLDL, also defined by its content of oxidized Ω-6. Flanagan’s cursory search of the literature misses a vast number of papers looking at health risks and outcomes in humans. For instance:
“Results: The results showed higher levels of Lp(a) in the serum of patients with psoriasis compared with controls (P<0·001). Higher levels of lipid hydroperoxides (P<0·001) and lower PON1 activity were observed in the serum of patients compared with healthy subjects, confirming that psoriasis is associated with oxidative stress. The imbalance between oxidative stress and antioxidant enzymes, and the increase of Lp(a) serum levels was related to the extent and severity of psoriasis. Finally, our results demonstrated that Lp(a) levels were positively correlated with markers of lipid peroxidation and negatively related to PON1 activity, suggesting that subjects with higher levels of Lp(a) are more exposed to oxidative damage.” (Ferretti et al., 2012)
This study doesn’t include the word “human” so Flanagan’s search missed it.
But, that’s not a trial. Psoriasis is widely recognized to be an inflammatory condition. Is there any reason to think that dietary manipulation of Ω-3 and Ω-6 might help it?
A RCT of a reduced-Ω-6, increased Ω-3 diet found just that (Guida, 2017).
So let’s look at one which also doesn’t seem to turn up in that PubMed search.
We’ve all heard of the Mediterranean diet for heart prevention and general health. The idea for that diet for coronary disease prevention came from Ancel Keys (Page, 1961, Keys, 1975), or, as Flanagan put it above, “it all started with Ancel Keys”.
“In the present trial of diet to reduce cardiac mortality and morbidity after myocardial infarction, we adapted a diet associated with a low mortality rate from coronary heart disease and all causes in the Seven Country Study.” (de Lorgeril, 1994)
The Seven Country Study was, of course, Keys’ famous epidemiological study looking at diet and heart disease (Verschuren, 1995). Somewhat unfairly, perhaps, de Lorgeril left the control patients in the care of the hospital:
“…control patients received no dietary advice apart from that of hospital dieticians or attending physicians.” (de Lorgeril, 1994)
Which was:
“…close to the prudent diet of the American Heart Association (total lipids, 31% energy; saturated fats, 10.5%; polyunsaturated/saturated ratio, 0.78).”
Unfairly, because when the results came out the control group did rather poorly.
In a way, this was a trial of Keys, as he was also instrumental in setting the foundation for the AHA prudent diet:
“A diet moderate in calories and fat (about 23-35 per cent of total calories from fat) may be helpful for these coronary-prone persons. Substitution of poly-unsaturated for a substantial part of the saturated fat in the diet may also be a valuable addition to this program.” (Page, 1961)
As with many of these diet trials in humans, many things changed. Unlike every other diet trial up to this point, one of the recommendations was to lower n-6 fat:
“We have reported that a dietary polyunsaturated to saturated fat ratio more than 1, even if associated with a decrease in plasma cholesterol, enhanced platelet aggregation to adenosine diphosphate. High platelet aggregation is associated with myocardial infarction and closely predicts coronary events. In addition, a high concentration of linoleic acid could be associated with increased lipid peroxidation and platelet-induced aggregation.” (de Lorgeril et al., 1994)
Flanagan explains the importance of the P:S ratio:
“The most important contribution from the [Ancel] Keys Equation was identification of the ‘P:S ratio’, i.e, the ratio of polyunsaturated to saturated fats in the diet, as the most important factor determining blood cholesterol levels in response to diet.”
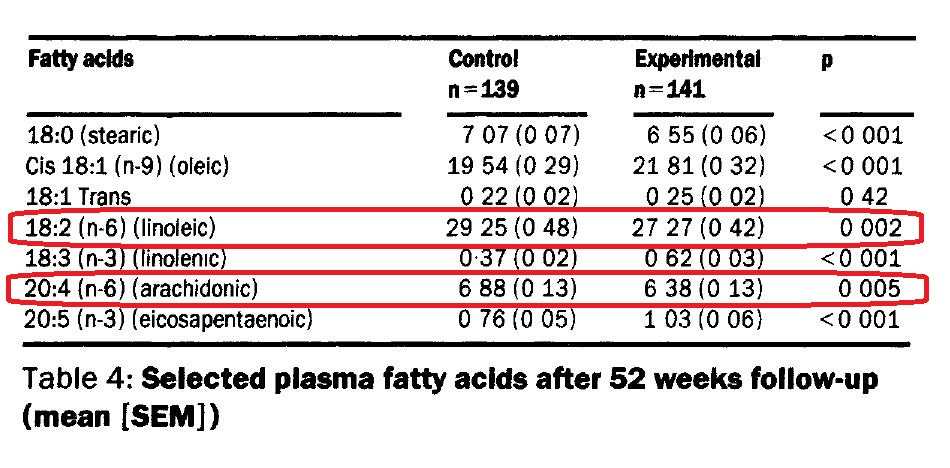
(de Lorgeril et al., 1994) specifically targeted a low P:S ratio, and a low level of linoleic acid. They also increased n-3 alpha-linolenic acid (ALA, see effect on CRP in (Bemelmans et al., 2004), above). Unfortunately, that was the part of the intervention that was featured in the title, and the reduction in LA has largely been ignored (detailed below).
(Incidentally, they also noted that arachidonic acid fell in the experimental group, see table 4, something Flanagan assured us doesn’t happen.)
The fallout of this study was enormous. The AHA got a black eye, and modified its standard recommendations somewhat:
“Several randomized controlled trials recently have demonstrated beneficial effects of both α-linolenic acid (76) and marine [Ω]-3 fatty acids (77, 78, 79) on both coronary morbidity and mortality in patients with coronary disease. Because of the beneficial effects of [Ω]-3 fatty acids on risk of coronary artery disease as well as other diseases such as inflammatory and autoimmune diseases, the current intake, which is generally low, should be increased.” (Krauss, 2001)
Footnote 76 was (de Lorgeril, 1999), the final report of what was now called the Lyon Diet Heart Study.
(Ironically, Ronald Krauss, the first author of the AHA paper went on to publish (Siri-Tarino, 2010), exonerating saturated fat.)
The New York Times of course covered the story, as it was a major development, noting:
“Those following the Mediterranean diet were 50 percent to 70 percent less likely than the comparison group to develop recurrent heart disease, including fatal and nonfatal heart attacks.” (Brody, 1999)
But, oddly, mischaracterized the comparison group diet:
“…and the other half were advised to eat a more traditional Western diet that was relatively low in fat, saturated fat and cholesterol.”
The description of the diet had changed. The first paper was published in The Lancet, with no affiliation to the AHA; the second appeared in the AHA’s Circulation journal, where it became a “prudent Western-type diet”.
Neither made any mention of the reduction in n-6 fats in the diet, and the AHA and the NYT continued to recommend a high Ω-6 seed oil intake.
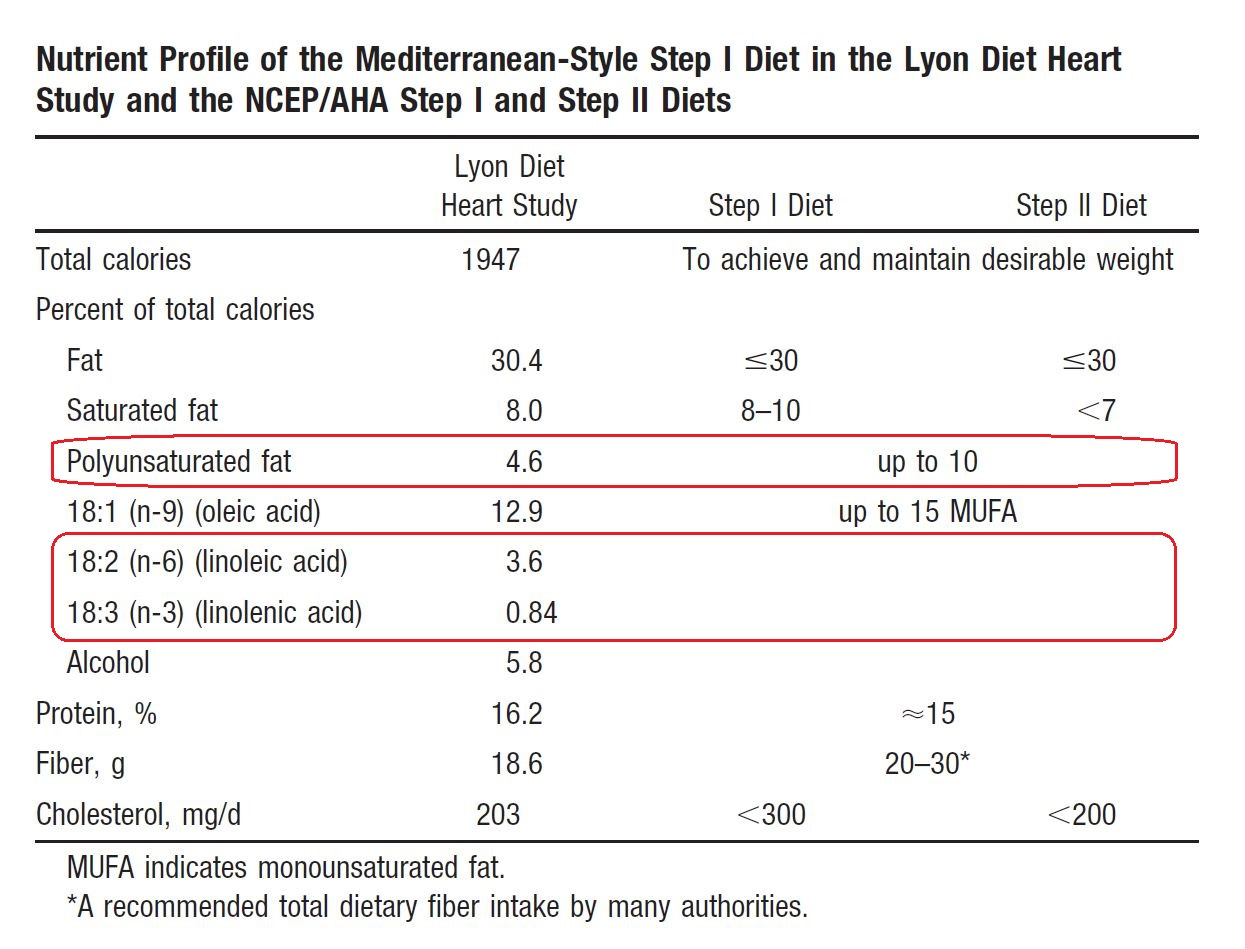
The AHA was so enamored of this study that the following year they published an “AHA Science Advisory” (Kris-Etherton, 2001) in which they compared the Lyon diet to the AHA diets.
In the table above, you can see that they note the amount of LA. Yet it’s ignored entirely in their recommended changes to their diet, and (de Lorgeril, 1994) with its extensive discussion of LA is also ignored entirely—it doesn’t even appear in the footnotes. Of course they probably would have argued that existing studies, like the Minnesota Coronary Experiment, discussed below, supported a beneficial role for Ω-6. Since the AHA had been promoting consumption of Ω-6 fats for cardiovascular disease prevention in this journal for decades, as discussed above (Page, 1961), this would have been quite the embarrassing about-face, with vast consequences for the American and world diet.
Meta-analyses either mischaracterize the study, or even better, just ignore it (Hooper, 2018, 2020).
So this study perfectly fits Flanagan’s description. It’s a trial, testing lipid peroxidation via modification of dietary polyunsaturated fats, in humans.
It’s also arguably the most famous diet-heart study of the last five decades.
He doesn’t even mention it, but concludes:
“…it appears that there is little evidence from controlled intervention studies that lipid peroxidation from PUFA intake, of various total PUFA intake and varying LA levels, is a major cause for concern in vivo.”
Unless you have heart disease, which is the leading cause of death.
He then discusses a few minor studies, which I’m going to skip, as they are mice compared to this elephant.
“Dusty old human studies; The oft-cited human studies.”
The logical fallacies come fast and hard in this section.
Flanagan set the stage with the “dusty old human studies” line, which implies that scientific papers have a sell-by date, or something. It’s a fundamentally anti-science position. The current dietary guidelines for essential fatty acids, for instance, depend on a paper from 1930 (Burr, 1930), which I just saw cited yesterday in an online presentation by a leading scientist in the field (Dyall, 2021). That’s 94 years old, and still considered valid for human health and nutrition. The “dusty old” line is just an attempt to discredit these studies without considering their merit, as disingenuous a rhetorical tactic as is possible.
So what’s he trying to hide?
He looks at two reanalyses of two old studies, the Sydney Diet-Heart Study (SDHS) ((Woodhill, 1978), reanalysis (Ramsden, 2013b)), and the Minnesota Coronary Experiment (MCE) ((Frantz, 1989), reanalysis (Ramsden, 2016b)). Now what’s very interesting in the context of this discussion is that the scientist who did both of these is none other than Christopher Ramsden, who did the two migraine studies mentioned above (Mann, 2018; Ramsden, 2013), demonstrating that dietary Ω-6 causes an inflammatory condition. He’s one of the leading researchers into Ω-6 in the world, and is currently heading the Lipid Peroxidation Unit at the National Institutes of Health.
Ramsden, a medical doctor as well as a scientist, is working on the most important health issue of our time—what is causing the pandemic of chronic diseases, which includes all our leading causes of death and disability, and he’s moving the needle. However, the idea that these problems may be iatrogenic (caused by the health authorities) seems to irk a lot of these authorities and people who aspire to that position. (Which is a big claim, but we’ve got evidence for it right in front of us. We’ll get to that below.)
SDHS
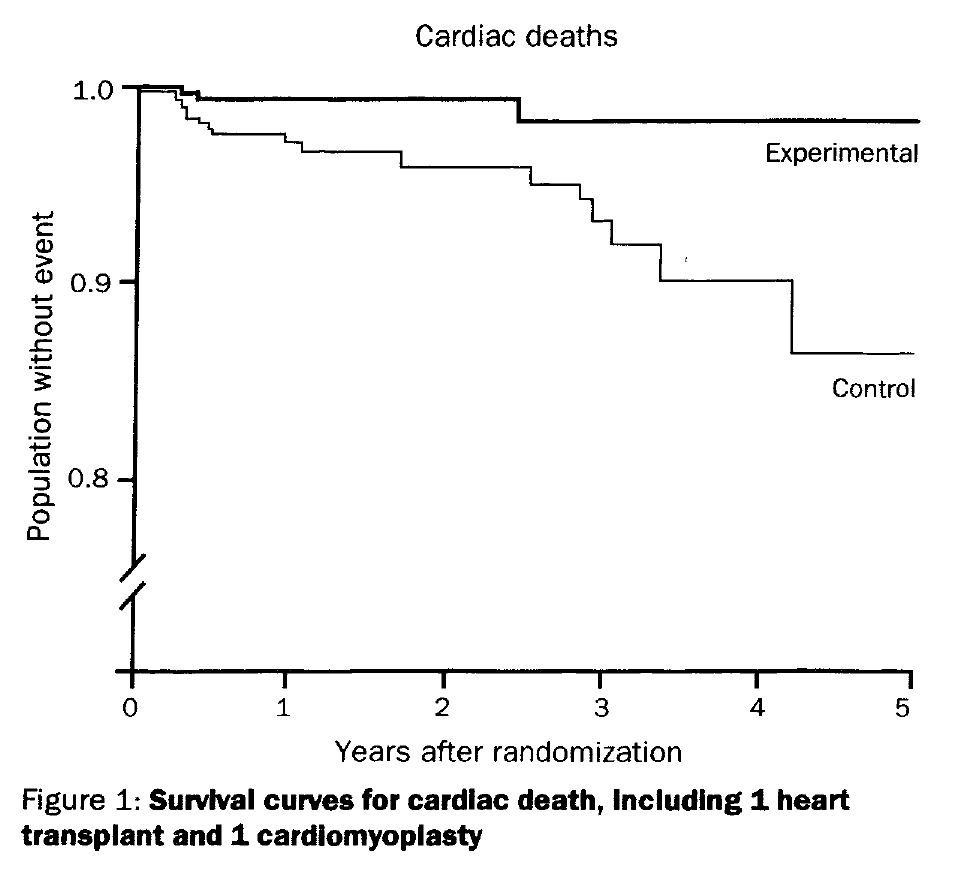
SDHS is important because it is one of a number of studies showing that the recommended dietary intervention to reduce heart disease in fact led to a higher mortality rate, despite lowering cholesterol levels.
Flanagan claims the study is “flawed” and thus not worthy of consideration. Without going through all his claims, his most significant one is:
“…the intervention group substantially increased PUFA intake with the provided foods, likely consuming substantial amounts of trans-fats – which are unequivocally associated with significant increased risk for CVD [(Mozaffarian, 2006)]. Moreover, attributing this effect to LA is not possible, because while the oil may have had a high LA content, margarines at the time [(Bernfeld, 1962)]were predominantly monounsaturated fats, together with the high trans-fat content.”
The references Flanagan provides for the trans fat contents of margarines, while contemporaneous, are from Massachusetts (Bernfeld, 1962) and Ohio (Brown, 1959; Mabrouk, 1956), they do not necessarily represent the diet used in the trial in Australia.
More importantly, Flanagan does not consider in his criticism of SDHS the immediate effects of trans fats on the human body. Yet he cites (Mozaffarian, 2006), where it is explained:
“...the consumption of trans fatty acids raises levels of low-density lipoprotein (LDL) cholesterol… Thus, trans fatty acids have markedly adverse effects on serum lipids.”
And he quotes (Page et al., 1957):
“These experiments showed that relatively unsaturated fats of vegetable or marine origin tend to lower while, per contra, the particular hydrogenated vegetable fats usually, and saturated animal fat rather regularly, tended to increase serum cholesterol.” [Emphasis added].
Critically, Flanagan does not address the efforts by the authors of the SDHS to create a diet that would have the desired effect in the trial. Ramsden et al. explain:
“Another factor that could have been altered by the intervention is dietary trans fatty acids, which are known to raise total and low density lipoprotein cholesterol... Restriction of common margarines and shortenings (major sources of trans fatty acids) in the intervention group would be expected to substantially reduce consumption of trans fatty acids compared with the control group.” (Ramsden, 2013b)
The effect of Ω-6 PUFA on cholesterol was well-known at the time (it’s why PUFA was recommended):
“It has also been established that substitution of polyunsaturated fatty acids for saturated fatty acids in the diet lowered serum cholesterol.” (Woodhill et al., 1978)
Which is what Flanagan noted above. So, since the authors were aware of this:
“Although the precise composition of this margarine was not specified, it was selected for the study because of its ability to lower blood cholesterol and its high PUFA to SFA ratio, two characteristics of margarines that contain comparatively low amounts of trans fatty acids.” (Ramsden, 2013b)
Thus we have a reliable biomarker to track intake of trans fats: the cholesterol levels of the dieters.
“Trans fats raise serum total and low-density lipoprotein (LDL) cholesterol. Thus, the significant reduction in serum cholesterol among the n-6 LA intervention group (-13%) compared to the control group (-5.5%) in this randomized controlled trial is not consistent with the premise that increased mortality in the intervention group was due to trans fat intake.” (Ramsden, 2013a)
So Flanagan’s contention that the results were due to the intervention group “…likely consuming substantial amounts of trans-fats…” appears to be refuted by the facts, and by his own citations!
Flanagan is clearly aware of this, since he devotes an entire section to this effect.
“Together, these observations and other factors discussed in our manuscript indicate that trans fats are not a convincing explanation for the increased risk of death in the n-6 LA intervention group.” (Ramsden, 2013b)
It’s unclear why he ignores this in his critique of this study, but it is clear that his critique is therefore invalid, if not a misdirection.
MCE
This is where Flanagan really goes off the rails. After stating:
“This also tends to come hand-in-hand with the “it all started with Ancel Keys” diatribe, regurgitated by automatons who haven’t even read his research, or even of the wider diet-heart literature, from that period.”
“…Citing studies like these [SDHS and MCE] involves either total cognitive dissonance or lack of scientific literacy…”
He repeatedly cites Keys’ work, clearly with approval. He then states: “Here is the thing: MCE was a poorly executed study by any standards.”
Poor Ancel Keys, who was a principal investigator and designer of the MCE!
The MCE was not a stand-alone investigation, but was a sub-set of the National Diet-Heart Study:
“The six principal investigators are Drs. Benjamin M. Raker, Ivan D. Frantz, Jr., Ancel Keys, Laurance W. Kinsell, Jeremiah Stamler, and Fredrick J. Stare.” (Baker et al., 1963)
Keys was a co-author on that paper. This was not a “poorly executed study”, in fact, it was the apotheosis of medical science:
“Within the last 10 years several investigators have undertaken studies to examine the influence of dietary modification on the occurrence of clinical coronary heart disease. By 1959 it was apparent that large-scale field trials might be feasible and that a national cooperative effort might be the appropriate means to accomplish this difficult research mission.
“In 1960, with the support of the National Heart Institute of the US Public Health Service, an Executive Committee on Diet and Heart Disease was established under the chairmanship of Dr. Irvine H. Page of Cleveland. This committee included leading medical, nutritional, and epidemiological scientists, as well as liaison representatives of the American Heart Association, the American Medical Association, the National Heart Institute, and the Nutrition Foundation.” (Baker et al., 1963)
This was a national effort to confirm Ancel Keys’ life work, in short.
Flanagan continues:
“Participants were not exclusively in-patients; many were in and out of hospitals, meaning that their participation in the intervention was not continuous, and they consumed study meals while in hospital, before returning to their day-to-day lives.”
This is an absurd critique, as all such large-scale studies are conducted on people out of the hospital, as it’s vastly too expensive to do such work on an in-patient basis. In fact, as the study designers state:
“The design of the feasibility study involves the recruitment of approximately 1,500 healthy, male volunteers. (Dr. Frantz's study will be conducted in a controlled, hospital environment; the other studies involve men living at home. )” (Baker et al., 1963)
So the MCE wing of the study, which wound up being much larger and of which Keys and Frantz were co-principal investigators, was intended to be the most rigorous part of the project.
Further, Flanagan is not making a new critique: Walter Willett, who took up Keys’ nutritional research mantle, made the same point (although he should have known better): “[MCE] was a major failure due to the massive dropouts and very short duration on the assigned diets” (Willett, 2016). Ramsden replied:
“Importantly, however, 2,355 MCE participants were exposed to MCE diets for ≥1 year. The original investigators emphasized this subsample, which alone is much larger than any other RCT testing the effects of replacing SFA with LA.” (Ramsden et al., 2016a)
Ironically, Willett lists his position as being the “Fredrick John Stare Professor of Epidemiology and Nutrition”. Stare was another principal investigator of the National Diet-Heart Study and co-author of (Baker et al., 1963). Claiming your admired predecessors are incompetent to discredit their old data is an odd, yet frequent, practice in medicine, often given without any thought to the credibility of the claimant.
Frantz, aside from being Keys’ colleague in Minnesota, was himself one of the top scientists in the field. As his obituary states:
“He ranks among the top 5% in history of NIH grant recipients…” (“Ivan D. Frantz Jr. Obituary,” 2009)
So I don’t know what Flanagan thinks to accomplish by impugning Frantz and Keys’ reputations. (Incidentally, Robert Franz, Ivan Frantz’s son, was a co-author of (Ramsden, 2016b), and is a cardiologist at the Mayo Clinic. He provided the records from his father’s estate that made this study possible.)
Flanagan, like Willett, seems desperate to disparage the MCE and this reanalysis; he continues:
“It is incorrect to state that the MCE is evidence of an increase in risk from PUFA, because the original trial findings were insignificant and spread across the null.”
This is just wrong, this is specifically addressed in (Amrhein et al., 2019), and in numerous other places in the statistical literature, although this erroneous assumption is widespread practice in medicine.
“The effect best supported by the data from a given experiment is always the observed effect, regardless of its significance.” (Goodman, 2008)
We know, for instance, that alcohol has both benefits and harms. The fact that that range of effects includes benefits does not allow one to dismiss the harms. He goes on at length in this erroneous vein.
“But even if we assumed for a minute that they were sound studies, they would still only be a handful of studies in the context of a wider literature accumulated from multiple lines of inquiry, from metabolic wards to prospective cohorts to RCTs, which all converge to support a benefit to PUFA.”
Flanagan here seems to misapprehend a basic fact of medicine.
First, you don’t weigh the literature with a scale. A metabolic ward study looking at the effect of LA on LDL cholesterol becomes irrelevant when you have a RCT in humans confirming the ward study, but showing an increased risk of death! Hard endpoints trump markers.
Second, rimonabant, a drug that conveyed a clear benefit for obesity in humans, was pulled from the market because it also induced a few people to commit suicide. Treatments are generally not allowed that convey a clear risk of harm, and what we seem to see from Ω-6 PUFA in multiple studies in humans is worse than rimonabant: no benefit, just harm.
Remember when he said this was what he wanted to see? Well, now he’s got them, and he seems desperate to ignore them!
Flanagan says:
“And, let’s not forgot both the anti-inflammatory effect of alpha-linolenic acid [ALA] and the anti-inflammatory role of EPA and DHA.”
An excellent point.
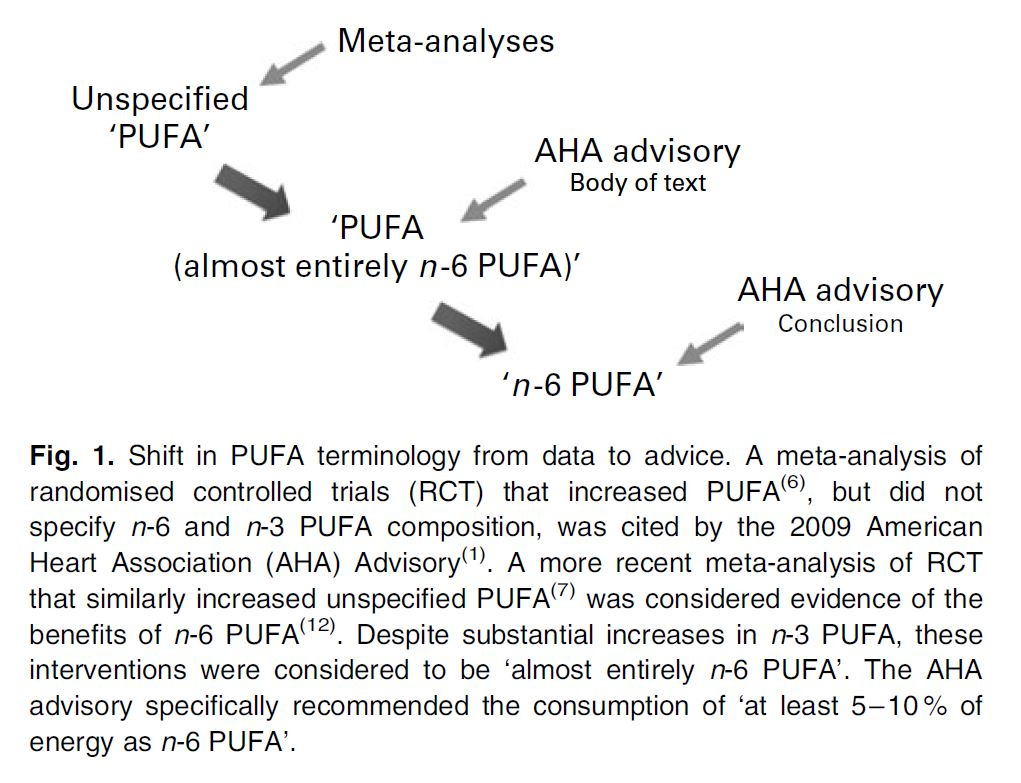
Ramsden et al. conducted a meta-analysis of Ω-6 vs Ω-3, which was what in part prompted the investigation into the SDHS and MCE (Ramsden et al., 2010), in light of the imprecise dietary advice given by the AHA.
“The AHA Science Advisory committee alternatively used the non-specific terms ‘PUFA’ and ‘PUFA (almost entirely n-6 PUFA)’ when referring to both individual RCT and a pooled analysis of six RCT cited in the body of the text(1,5,6) (Fig. 1). However, the advisory used the more specific term ‘n-6 PUFA’ in concluding that ‘at least 5–10% of energy from n-6 PUFA reduces the risk of CH relative to lower intakes.” (Ramsden, 2010)
They found:
“This analysis of RCT showed that mixed n-3/n-6 PUFA and n-6 specific PUFA diets have significantly different effects on CHD risk. In pooled analyses, mixed n-3/n-6 PUFA RCT significantly reduced the risks of non-fatal MI by 27% and non-fatal MI + CHD death by 22 %. By contrast, n-6 specific PUFA diets increased risk of all CHD endpoints, with the increased risk of death from all causes approaching statistical significance (Table 5).” (Ramsden, 2010)
(They updated that meta-analysis in light of the MCE findings in (Ramsden et al., 2016b Appendix 2))
The MCE is especially important because it was run by the man who created the hypothesis, with the entire medical infrastructure of the U.S. behind it, and it failed. While showing a decline in cholesterol, it did not translate to a benefit in mortality, but instead made mortality worse.
Conclusion
Flanagan:
“And we can look at the Hooper et al. updated meta-analysis [(Hooper et al., 2020)] of intervention studies from this year, which found that the replacement of SFA with PUFA was associated with a 27% [RR 0.73, 95% CI 0.58-0.92] reduction in risk for CVD events.”
Here we go again. This is cherry-picking. What the paper said:
“All‐cause mortality… There was little or no effect, regardless of what nutrients were used to replace the saturated fat removed, including replacement with PUFA, MUFA, CHO and/or protein (Analysis 1.9).” (Hooper et al., 2020)
This is Flanagan’s evidence to show “a benefit to PUFA.” While Hooper performs a poor analysis, and invalidly excludes a few of the crucial studies, their conclusion broadly agrees with that of (Ramsden et al., 2016b Appendix 2)—that the current recommendations of high Ω-6, while reducing cholesterol, does not convey the mortality benefit that was assumed.
The point of these dietary interventions is to avoid death. Nobody cares about their cholesterol level, we only care about the likelihood of dying earlier—although your cardiologist would probably prefer that you die from something other than heart disease!
In light of the Lyon Diet Heart Study, and the evidence of lower Ω-6 fat intakes historically prior to the current pandemic of heart disease, it’s reasonable to question the hypothesis, at the least. Given the indications of harm in multiple studies examining various chronic diseases, we need to start treating Ω-6–rich seed oils like we do synthetic trans fats, as a hazard.
Flanagan claims that pointing the finger at Keys’ work is invalid, “…the “it all started with Ancel Keys” diatribe, regurgitated by automatons who haven’t even read his research…” Flanagan is again misrepresenting the facts. Keys was indeed a crucial player in this line of research. As his protégé Dr. Henry Blackburn, who took over Keys’ lab, explained in introducing the Ancel Keys Memorial Lecture at the AHA:
“Thus, Keys basically "started it all"—clinical studies, laboratory diet experiments, population surveys and their ecological analyses, longitudinal cohort studies, and preventive trials. He brought the field together, building a new physiological, experimental, and epidemiological science as he went along that he called "physiological hygiene."… Few people realize Keys' remarkable contribution to international cardiology through his teamwork with White and other distinguished cardiologists.” (Blackburn, 1991)
It's important to understand that Keys’ two crucial studies to confirm his Diet-Heart Hypothesis both ended in failure. Keys was not listed as an author on the final reports from the MCE, as discussed above, or the Seven Country Study, which concluded:
“Therefore, from a public health perspective it is not enough to focus solely on serum cholesterol levels to decrease the burden of CHD in populations. It appears that reductions in serum total cholesterol levels are not likely to bring cultures with a high CHD risk, such as the United States and Northern Europe, back to a CHD mortality level characteristic for the Mediterranean and Japanese cultures unless other factors are also changed. The Mediterranean and Japanese diets, low in saturated fat and rich in antioxidants, may have beneficial effects both on the oxidizability of LDL particles and on thrombogenesis, apart from an effect on LDL levels per se. This stresses the importance of factors other than serum cholesterol, blood pressure, and smoking status, such as diet, in CHD prevention.” (Verschurenet al., 1995)
The Lyon Diet Heart study, Ramsden et al.’s work, and the vast literature looking at what factor actually induces dietarily-induced atherosclerosis point the finger at the dietary change that ought to be explored.
In light of Flanagan's legal background, this reminds me of an old legal aphorism:
“If the facts are against you, argue the law. If the law is against you, argue the facts. If the law and the facts are against you, pound the table and yell like hell” ― Carl Sandburg
Flanagan has clearly chosen the last approach. It’s time we replace bluster, misdirection, and preconceptions with a serious consideration of the evidence.
References
CRP image By Deposition authors: Ramadan, M.A., Shrive, A.K., Holden, D., Myles, D.A., Volanakis, J.E., DeLucas, L.J., Greenhough, T.J.;visualization author: User:Astrojan - http://www.rcsb.org/pdb/explore/explore.do?structureId=1lj7, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=48602470
OxLDL image from (Itabe et al., 2011)
References:
Abbey, M., Belling, G. B., Noakes, M., Hirata, F., & Nestel, P. J. (1993). Oxidation of low-density lipoproteins: Intraindividual variability and the effect of dietary linoleate supplementation. The American Journal of Clinical Nutrition, 57(3), 391–398. https://doi.org/10.1093/ajcn/57.3.391
Amrhein, V., Greenland, S., & McShane, B. (2019). Scientists rise up against statistical significance. Nature, 567(7748), 305. https://doi.org/10.1038/d41586-019-00857-9
Baker, B. M., Frantz, I. D., Jr., Keys, A., Kinsell, L. W., Page, I. H., Stamler, J., & Stare, F. J. (1963). The National Diet-Heart Study: An Initial Report. JAMA, 185(2), 105–106. https://doi.org/10.1001/jama.1963.03060020065024
Bemelmans, W. J. E., Lefrandt, J. D., Feskens, E. J. M., Haelst, P. L. van, Broer, J., Meyboom-de Jong, B., May, J. F., Tervaert, J. W. C., & Smit, A. J. (2004). Increased α -linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. European Journal of Clinical Nutrition, 58(7), 1083–1089. https://doi.org/10.1038/sj.ejcn.1601938
Bernfeld, P., Homburger, F., & Kelley, T. F. (1962). Fatty Acid Contents of Margarines and Other Table Fats. The American Journal of Clinical Nutrition, 11(6), 554–558. https://doi.org/10.1093/ajcn/11.6.554
Bhavadharini, B., Dehghan, M., Mente, A., Rangarajan, S., Sheridan, P., Mohan, V., Iqbal, R., Gupta, R., Lear, S., Wentzel-Viljoen, E., Avezum, A., Lopez-Jaramillo, P., Mony, P., Varma, R. P., Kumar, R., Chifamba, J., Alhabib, K. F., Mohammadifard, N., Oguz, A., … Yusuf, S. (2020). Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Research and Care, 8(1), e000826. https://doi.org/10.1136/bmjdrc-2019-000826
Blackburn, H. (1991). Introduction to Ancel Keys Lecture. Ancel Keys, pioneer. Circulation, 84(3), 1402–1404. https://doi.org/10.1161/01.CIR.84.3.1402
Brody, J. E. (1999, March 23). PERSONAL HEALTH; Savory Diet That’s Good For Heart? Let’s Eat. New York Times. https://query.nytimes.com/gst/fullpage.html?res=9B00E4DC1131F930A15750C0A96F958260
Brown, J. B. (1959). Changes in Nutritive Value of Food Fats during Processing and Cooking. Nutrition Reviews, 17(11), 321–325. https://doi.org/10.1111/j.1753-4887.1959.tb03553.x
Burr, G. O., & Burr, M. M. (1930). On the Nature and Rôle of the Fatty Acids Essential in Nutrition. Journal of Biological Chemistry, 86(2), 587–621. https://doi.org/10.1016/S0021-9258(20)78929-5
Chang, M.-K., Binder, C. J., Torzewski, M., & Witztum, J. L. (2002). C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proceedings of the National Academy of Sciences of the United States of America, 99(20), 13043–13048. https://doi.org/10.1073/pnas.192399699
Cordain, L. (2010). The Paleo Diet: Lose Weight and Get Healthy by Eating the Foods You Were Designed to Eat. Wiley. https://www.google.com/books/edition/The_Paleo_Diet/M5nLjwEACAAJ?hl=en
Cordain, L., Miller, J. B., Eaton, S. B., & Mann, N. (2000). Macronutrient estimations in hunter-gatherer diets. The American Journal of Clinical Nutrition, 72(6), 1589–1590. https://doi.org/10.1093/ajcn/72.6.1589
de Lorgeril, M., Renaud, S., Mamelle, N., Salen, P., Martin, J. L., Monjaud, I., Guidollet, J., Touboul, P., & Delaye, J. (1994). Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet (London, England), 343(8911), 1454–1459. https://doi.org/10.1016/s0140-6736(94)92580-1
de Lorgeril, M., Salen, P., Martin, J.-L., Monjaud, I., Delaye, J., & Mamelle, N. (1999). Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications After Myocardial Infarction. Circulation, 99(6), 779–785. https://doi.org/10.1161/01.CIR.99.6.779
Deleanu, M., Sanda, G. M., Stancu, C. S., Popa, M. E., & Sima, A. V. (2016). Profiles of Fatty Acids and the Main Lipid Peroxidation Products of Human Atherogenic Low Density Lipoproteins. Revista De Chimie, 67(1), 8–12. http://www.revistadechimie.ro/article_eng.asp?ID=4799
Doolen, S., Keyes, G. S., & Ramsden, C. E. (2020). Hydroxy-epoxide and keto-epoxide derivatives of linoleic acid activate trigeminal neurons. Neurobiology of Pain, 7, 100046. https://doi.org/10.1016/j.ynpai.2020.100046
Dyall, S. (2021, June 15). Bioactive lipids: An overview of their structure and functions [Educational]. Lipids Online Webinar, ISSFAL. https://www.issfal.org/index.php?option=com_jevents&task=icalrepeat.detail&evid=4&Itemid=1&year=2021&month=06&day=16&title=lipids-online-webinar-bioactive-lipids-an-overview-of-their-structure-and-functions&uid=4cb6904b107fc699d4d1a737958bbc67
Eaton, S. B. (1992). Humans, lipids and evolution. Lipids, 27(10), 814–820. https://doi.org/10.1007/BF02535856
Eaton, S. B., & Konner, M. (1985). Paleolithic Nutrition. New England Journal of Medicine, 312(5), 283–289. https://doi.org/10.1056/NEJM198501313120505
Edvinsson, L., Haanes, K. A., & Warfvinge, K. (2019). Does inflammation have a role in migraine? Nature Reviews Neurology, 15(8), 483–490. https://doi.org/10.1038/s41582-019-0216-y
Ferretti, G., Bacchetti, T., Campanati, A., Simonetti, O., Liberati, G., & Offidani, A. (2012). Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: Role of the enzyme paraoxonase-1. The British Journal of Dermatology, 166(1), 204–207. https://doi.org/10.1111/j.1365-2133.2011.10539.x
Flanagan, A. (2020a). About [Advertisement]. Alinea Nutrition. https://www.alineanutrition.com/about/
Flanagan, A. (2020b, September 26). Of Rats and Sydney Diet-Heart: Drawing a Line Under Polyunsaturated Pseudoscience [Blog]. Alinea Nutrition. https://www.alineanutrition.com/2020/09/26/of-rats-and-sydney-diet-heart-drawing-a-line-under-polyunsaturated-pseudoscience/
Frantz, I. D., Dawson, E. A., Ashman, P. L., Gatewood, L. C., Bartsch, G. E., Kuba, K., & Brewer, E. R. (1989). Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis (Dallas, Tex.), 9(1), 129–135. https://doi.org/10.1161/01.atv.9.1.129
Goodman, S. (2008). A Dirty Dozen: Twelve P-Value Misconceptions. Seminars in Hematology, 45(3), 135–140. https://doi.org/10.1053/j.seminhematol.2008.04.003
Gornoski, D. (2021, May 24). Omega 6 Linoleic Acid Research with Prof. Bruce Hammock, Prof. Bruce German, and Tucker Goodrich (No. 5/24/2021) [Mp3]. https://aneighborschoice.com/omega-6-linoleic-acid-research-with-prof-bruce-hammock-prof-bruce-german-and-tucker-goodrich/
Guida, B., Napoleone, A., Trio, R., Nastasi, A., Balato, N., Laccetti, R., & Cataldi, M. (2014). Energy-Restricted, N-3 Polyunsaturated Fatty Acids-Rich Diet Improves the Clinical Response to Immuno-Modulating Drugs in Obese Patients with Plaque-Type Psoriasis: A Randomized Control Clinical Trial. Clinical Nutrition (Edinburgh, Scotland), 33(3), 399–405. https://doi.org/10.1016/j.clnu.2013.09.010
Hao, S., Ji, J., Zhao, H., Shang, L., Wu, J., Li, H., Qiao, T., & Li, K. (2015). Mitochondrion-Targeted Peptide SS-31 Inhibited Oxidized Low-Density Lipoproteins-Induced Foam Cell Formation through both ROS Scavenging and Inhibition of Cholesterol Influx in RAW264.7 Cells. Molecules (Basel, Switzerland), 20(12), 21287–21297. https://doi.org/10.3390/molecules201219764
Hargrove, R. L., Etherton, T. D., Pearson, T. A., Harrison, E. H., & Kris-Etherton, P. M. (2001). Low Fat and High Monounsaturated Fat Diets Decrease Human Low Density Lipoprotein Oxidative Susceptibility In Vitro. The Journal of Nutrition, 131(6), 1758–1763. https://doi.org/10.1093/jn/131.6.1758
Hooper, L., Al‐Khudairy, L., Abdelhamid, A. S., Rees, K., Brainard, J. S., Brown, T. J., Ajabnoor, S. M., O’Brien, A. T., Winstanley, L. E., Donaldson, D. H., Song, F., & Deane, K. H. (2018). Omega‐6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 7. https://doi.org/10.1002/14651858.CD011094.pub3
Hooper, L., Martin, N., Jimoh, O. F., Kirk, C., Foster, E., & Abdelhamid, A. S. (2020). Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews, 5. https://doi.org/10.1002/14651858.CD011737.pub2
Itabe, H., Obama, T., & Kato, R. (2011). The Dynamics of Oxidized LDL during Atherogenesis. Journal of Lipids, 2011, e418313. https://doi.org/10.1155/2011/418313
Ivan D. Frantz Jr. Obituary. (2009, January 25). Pioneer Press. Legacy.com. https://www.legacy.com/us/obituaries/twincities/name/ivan-frantz-obituary?pid=123233693
Kennedy, D. J., Kuchibhotla, S., Westfall, K. M., Silverstein, R. L., Morton, R. E., & Febbraio, M. (2011). A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovascular Research, 89(3), 604–613. https://doi.org/10.1093/cvr/cvq360
Keys, A., & Keys, M. (1975). How to Eat Well and Stay Well the Mediterranean Way. Doubleday. https://www.google.com/books/edition/How_to_Eat_Well_and_Stay_Well_the_Medite/Lt6nQAAACAAJ?hl=en
Kodani, S. D., & Hammock, B. D. (2015). The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: Drug metabolism to therapeutics for chronic pain. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 43(5), 788–802. https://doi.org/10.1124/dmd.115.063339
Krauss, R. M., Eckel, R. H., Howard, B., Appel, L. J., Daniels, S. R., Deckelbaum, R. J., Erdman, J. W., Jr, Kris-Etherton, P., Goldberg, I. J., Kotchen, T. A., Lichtenstein, A. H., Mitch, W. E., Mullis, R., Robinson, K., Wylie-Rosett, J., St. Jeor, S., Suttie, J., Tribble, D. L., & Bazzarre, T. L. (2001). AHA Scientific Statement: AHA Dietary Guidelines: Revision 2000: A Statement for Healthcare Professionals From the Nutrition Committee of the American Heart Association. The Journal of Nutrition, 131(1), 132–146. https://doi.org/10.1093/jn/131.1.132
Kris-Etherton, P. M., Eckel, R. H., Howard, B. V., Jeor, S. C. S., & Bazzarre, T. L. (2001). AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation, 103(13), 1823–1825. https://doi.org/10.1161/01.CIR.103.13.1823
Lieberman, D. (2013). The Story of the Human Body: Evolution, Health, and Disease. Knopf Doubleday Publishing Group. https://www.penguinrandomhouse.com/books/206671/the-story-of-the-human-body-by-daniel-e-lieberman/
Lieberman, D. E., Krovitz, G. E., Yates, F. W., Devlin, M., & St. Claire, M. (2004). Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. Journal of Human Evolution, 46(6), 655–677. https://doi.org/10.1016/j.jhevol.2004.03.005
Mabrouk, A. F., & Brown, J. B. (1956). The trans fatty acids of margarines and shortenings. Journal of the American Oil Chemists Society, 33(3), 98–102. https://doi.org/10.1007/BF02632289
Mann, J. D., Faurot, K. R., MacIntosh, B., Palsson, O. S., Suchindran, C. M., Gaylord, S. A., Lynch, C., Johnston, A., Maiden, K., Barrow, D. A., Hibbeln, J. R., & Ramsden, C. E. (2018). A sixteen-week three-armed, randomized, controlled trial investigating clinical and biochemical effects of targeted alterations in dietary linoleic acid and n-3 EPA+DHA in adults with episodic migraine: Study protocol. Prostaglandins, Leukotrienes and Essential Fatty Acids, 128, 41–52. https://doi.org/10.1016/j.plefa.2017.11.002
Micha, R., & Mozaffarian, D. (2010). Saturated Fat and Cardiometabolic Risk Factors, Coronary Heart Disease, Stroke, and Diabetes: A Fresh Look at the Evidence. Lipids, 45(10), 893–905. https://doi.org/10.1007/s11745-010-3393-4
Mozaffarian, D., Katan, M. B., Ascherio, A., Stampfer, M. J., & Willett, W. C. (2006). Trans Fatty Acids and Cardiovascular Disease. New England Journal of Medicine, 354(15), 1601–1613. https://doi.org/10.1056/NEJMra054035
National Research Council. (2000). Chapter 7 Using Model Animals to Assess and Understand Developmental Toxicity. In Scientific Frontiers in Developmental Toxicology and Risk Assessment. National Academies Press (US). https://doi.org/10.17226/9871
Norris, A. L., Steinberger, J., Steffen, L. M., Metzig, A. M., Schwarzenberg, S. J., & Kelly, A. S. (2011). 7.04 Circulating Oxidized LDL and Inflammation in Extreme Pediatric Obesity. Obesity, 19(7), 1415–1419. https://doi.org/10.1038/oby.2011.21
Page, I. H., Allen, E. V., Chamberlain, F. L., Keys, A., Stamler, J., & Stare, F. J. (1961). Dietary Fat and Its Relation to Heart Attacks and Strokes. Circulation, 23(1), 133–136. https://doi.org/10.1161/01.CIR.23.1.133
Page, I. H., Stare, F. J., Corcoran, A. C., Pollack, H., & Wilkinson, C. F. (1957). Atherosclerosis and the Fat Content of the Diet. Circulation, 16(2), 163–178. https://doi.org/10.1161/01.CIR.16.2.163
Parthasarathy, S., Khoo, J. C., Miller, E., Barnett, J., Witztum, J. L., & Steinberg, D. (1990). Low density lipoprotein rich in oleic acid is protected against oxidative modification: Implications for dietary prevention of atherosclerosis. Proceedings of the National Academy of Sciences, 87(10), 3894–3898. https://doi.org/10.1073/pnas.87.10.3894
Peat, R. (2006). About Ray Peat [Advertisement]. Ray Peat. http://raypeat.com/about.shtml
Peat, R. (2007). The Great Fish Oil Experiment [Blog]. Ray Peat. https://raypeat.com/articles/articles/fishoil.shtml
Pradalier, A., Bakouche, P., Baudesson, G., Delage, A., Cornaille-Lafage, G., Launay, J. M., & Biason, P. (2001). Failure of omega-3 polyunsaturated fatty acids in prevention of migraine: A double-blind study versus placebo. Cephalalgia: An International Journal of Headache, 21(8), 818–822. https://doi.org/10.1046/j.1468-2982.2001.218240.x
Que, X., Hung, M.-Y., Yeang, C., Gonen, A., Prohaska, T. A., Sun, X., Diehl, C., Määttä, A., Gaddis, D. E., Bowden, K., Pattison, J., MacDonald, J. G., Ylä-Herttuala, S., Mellon, P. L., Hedrick, C. C., Ley, K., Miller, Y. I., Glass, C. K., Peterson, K. L., … Witztum, J. L. (2018). Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature, 558(7709), 301–306. https://doi.org/10.1038/s41586-018-0198-8
Ramsden, C. E., Faurot, K. R., Zamora, D., Suchindran, C. M., MacIntosh, B. A., Gaylord, S., Ringel, A., Hibbeln, J. R., Feldstein, A. E., Mori, T. A., Barden, A., Lynch, C., Coble, R., Mas, E., Palsson, O., Barrow, D. A., & Mann, D. J. (2013). Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain, 154(11), 2441–2451. https://doi.org/10.1016/j.pain.2013.07.028
Ramsden, C. E., Hibbeln, J. R., Majchrzak, S. F., & Davis, J. M. (2010). n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: A meta-analysis of randomised controlled trials. The British Journal of Nutrition, 104(11), 1586–1600. https://doi.org/10.1017/S0007114510004010
Ramsden, C. E., Zamora, D., Leelarthaepin, B., Majchrzak-Hong, S. F., Faurot, K. R., Suchindran, C. M., Ringel, A., Davis, J. M., & Hibbeln, J. R. (2013a). Re: Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ, 346, e8707. https://doi.org/10.1136/bmj.e8707
Ramsden, C. E., Zamora, D., Leelarthaepin, B., Majchrzak-Hong, S. F., Faurot, K. R., Suchindran, C. M., Ringel, A., Davis, J. M., & Hibbeln, J. R. (2013b). Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ, 346. https://doi.org/10.1136/bmj.e8707
Ramsden, C. E., Zamora, D., Majchrzak-Hong, S. F., Faurot, K. R., Broste, S. K., Frantz, R. P., Davis, J. M., Ringel, A., Suchindran, C. M., & Hibbeln, J. R. (2016a). Re: Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ, 2016(353), i1246. https://doi.org/10.1136/bmj.i1246
Ramsden, C. E., Zamora, D., Majchrzak-Hong, S., Faurot, K. R., Broste, S. K., Frantz, R. P., Davis, J. M., Ringel, A., Suchindran, C. M., & Hibbeln, J. R. (2016b). Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ, 353. https://doi.org/10.1136/bmj.i1246
Reaven, P. D., Grasse, B. J., & Tribble, D. L. (1994). Effects of linoleate-enriched and oleate-enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arteriosclerosis and Thrombosis: A Journal of Vascular Biology, 14(4), 557–566. https://doi.org/10.1161/01.atv.14.4.557
Rett, B. S., & Whelan, J. (2011). Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutrition & Metabolism, 8, 36. https://doi.org/10.1186/1743-7075-8-36
Rice, M. E. (2020). Bruce D. Hammock: Science Should Be Fun. American Entomologist, 66(1), 14–19. https://doi.org/10.1093/ae/tmaa010
Shapira, N., & Pinchasov, J. (2008). Modified Egg Composition To Reduce Low-Density Lipoprotein Oxidizability: High Monounsaturated Fatty Acids and Antioxidants versus Regular High n−6 Polyunsaturated Fatty Acids. Journal of Agricultural and Food Chemistry, 56(10), 3688–3693. https://doi.org/10.1021/jf073549r
Singh, S. K., & Agrawal, A. (2019). Functionality of C-Reactive Protein for Atheroprotection. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.01655
Siri-Tarino, P. W., Sun, Q., Hu, F. B., & Krauss, R. M. (2010). Saturated fat, carbohydrate, and cardiovascular disease. The American Journal of Clinical Nutrition, 91(3), 502–509. https://doi.org/10.3945/ajcn.2008.26285
Spiteller, D., & Spiteller, G. (2000). Oxidation of Linoleic Acid in Low-Density Lipoprotein: An Important Event in Atherogenesis. Angewandte Chemie International Edition, 39(3), 585–589. https://doi.org/10.1002/(SICI)1521-3773(20000204)39:3<585::AID-ANIE585>3.0.CO;2-G
Stiekema, L. C. A., Stroes, E. S. G., Verweij, S. L., Kassahun, H., Chen, L., Wasserman, S. M., Sabatine, M. S., Mani, V., & Fayad, Z. A. (2019). Persistent arterial wall inflammation in patients with elevated lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. European Heart Journal, 40(33), 2775–2781. https://doi.org/10.1093/eurheartj/ehy862
Su, H., Liu, R., Chang, M., Huang, J., & Wang, X. (2017). Dietary linoleic acid intake and blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Food & Function, 8(9), 3091–3103. https://doi.org/10.1039/c7fo00433h
U.S. Food & Drug Administration. (2016, November 18). Import Alert 26-04: Detention Without Physical Examination of Expressed Mustard Oil [Regulation]. FDA. https://www.accessdata.fda.gov/cms_ia/importalert_89.html
van der Valk Fleur M., Bekkering Siroon, Kroon Jeffrey, Yeang Calvin, Van den Bossche Jan, van Buul Jaap D., Ravandi Amir, Nederveen Aart J., Verberne Hein J., Scipione Corey, Nieuwdorp Max, Joosten Leo A.B., Netea Mihai G., Koschinsky Marlys L., Witztum Joseph L., Tsimikas Sotirios, Riksen Niels P., & Stroes Erik S.G. (2016). Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation, 134(8), 611–624. https://doi.org/10.1161/CIRCULATIONAHA.116.020838
Verschuren, W. M. M., Jacobs, D. R., Bloemberg, B. P. M., Kromhout, D., Menotti, A., Aravanis, C., Blackburn, H., Buzina, R., Dontas, A. S., Fidanza, F., Karvonen, M. J., Nedelijković, S., Nissinen, A., & Toshima, H. (1995). Serum Total Cholesterol and Long-term Coronary Heart Disease Mortality in Different Cultures: Twenty-five—Year Follow-up of the Seven Countries Study. JAMA, 274(2), 131–136. https://doi.org/10.1001/jama.1995.03530020049031
Watson, J., Jones, H. E., Banks, J., Whiting, P., Salisbury, C., & Hamilton, W. (2019). Use of multiple inflammatory marker tests in primary care: Using Clinical Practice Research Datalink to evaluate accuracy. The British Journal of General Practice, 69(684), e462–e469. https://doi.org/10.3399/bjgp19X704309
Willeit, P., Yeang, C., Moriarty, P. M., Tschiderer, L., Varvel, S. A., McConnell, J. P., & Tsimikas, S. (2020). Low-Density Lipoprotein Cholesterol Corrected for Lipoprotein(a) Cholesterol, Risk Thresholds, and Cardiovascular Events. Journal of the American Heart Association, 9(23), e016318. https://doi.org/10.1161/JAHA.119.016318
Willett, W. C. (2016). Re: Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ, 2016(353), i1246. https://doi.org/10.1136/bmj.i1246
Witztum, J. L. (2002). Minimally Modified LDL Binds to CD14, Induces Macrophage Spreading via TLR4/MD-2, and Inhibits Phagocytosis of Apoptotic Cells. JBC : Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M209634200
Witztum, J. L., & Steinberg, D. (1991). Role of oxidized low density lipoprotein in atherogenesis. Journal of Clinical Investigation, 88(6), 1785–1792. https://doi.org/10.1172/JCI115499
Woodhill, J. M., Palmer, A. J., Leelarthaepin, B., McGilchrist, C., & Blacket, R. B. (1978). Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Advances in Experimental Medicine and Biology, 109, 317–330. https://doi.org/10.1007/978-1-4684-0967-3_18
Zhang, Y., Wei, J., Wang, F., Chen, M., & Zhang, M. (2012). Elevated Levels of Oxidized Low-Density Lipoprotein Correlate Positively with C-Reactive Protein in Patients with Acute Coronary Syndrome. Cell Biochemistry and Biophysics, 62(2), 365–372. https://doi.org/10.1007/s12013-011-9295-0
Zink, K. D., & Lieberman, D. E. (2016). Impact of meat and Lower Palaeolithic food processing techniques on chewing in humans. Nature, 531(7595), 500–503. https://doi.org/10.1038/nature16990









Wow. It took me 3 days to get through this (it was a busy weekend), but it was well worth the read. It would have been much easier to just say "This dude is a liar and an idiot." but I believe your approach is way better.
I like to click the linked studies and at least pretend like I'm reading through them, so I did that with one of the references from the source article only to be met with a bad link. Then you explain that it is the original link from the article and it doesn't actually exist. Do people just assume you won't check things and verify what they write?