Seed Oil Toxins Are Absorbed From Food
tl;dr: They're toxic on multiple levels and they should be avoided.
A Question
“The missing link for me is this: Are there studies showing that increasing dietary HNE consumption increases HNE levels in our bodies?”
It’s well-known that seed oils are fragile and subject to oxidation—actually peroxidation, but we’re going to keep it simple here.
Bing and Google both return the phrase “lipid peroxidation” when searching for peroxidation.
“Cell damage” is the part that we are concerned about, of course!
The unsaturated fats are actually just polyunsaturated fats (PUFA), as mono-unsaturated fats (MUFA) are much less likely to oxidize, and are not nearly as toxic.
A Qualifier
To answer this question, a couple of observations must be made. I have long used HNE as a short-hand for oxidized Ω-6 fats, and I am presuming that the question is asked in that context.
There are many forms of oxidized Ω-6 fats, only some of which are included in the diagram above.
The most-studied toxic product of seed oil consumption is HNE (also 4-HNE, 4-hydroxy-2-nonenal) (Schaich, 2020). It’s involved in a variety of different human disease processes, including obesity, diabetes, heart disease, cancer (where it was first discovered), fatty liver disease, auto-immune conditions, neurodegenerative diseases (like Alzheimer’s), and all the other chronic diseases I have looked at (Spiteller, 1998). It’s only produced from Ω-6 fats like those in seed oils (linoleic acid), and while it can also be produced from Ω-6 fats from animals (also arachidonic acid), since we eat much more seed oils now than animal fats (Lee, 2022), that is the primary source of HNE (Spiteller, 1998).
HNE can be produced in fats during cooking, either deep-frying (Le Gresley, 2021) or pan-frying (Grootveld, 2014), with the latter being worse, since the more of the shallow oil is exposed to oxygen. HNE is then absorbed into the foods cooked in the fat (Seppanen, 2004).
It’s important to recognize that if a precursor for HNE is ingested, including either of the two main dietary Ω-6 fatty acids, they can degrade into HNE or any of the other toxic Ω-6 metabolites. And some of the other metabolites are even more toxic than HNE. So if one oxidative product is seen, it is to be assumed that the others are also present.
Rancid Fats
The common word for lipid peroxidation is “rancid”, meaning the fats have become oxidized. It’s long been known that foods that go rancid are unhealthy. Your grandmother would know to smell a fish to see if it was still good, the odor that would alert her that it had gone bad, become rancid, would be the oxidized Ω-3 PUFAs in the fish.
In the 1940s, the U.S. Army did tests of rancid food items on various creatures, no doubt to figure out food storage limitations.
“Whipple fed four dogs a diet containing 25 percent rancid lard and produced an ‘oxidized fat syndrome’. The symptoms were loss of hair, skin lesions, emaciation, intestinal hemorrhage and death. Three dogs fed fresh fat remained normal.” (Committee on Food Research, 1945)
They noted that rancid fats promoted tumor formation, and they also noted that the increased calorie intake promoted by unspecified non-rancid fats also seemed to promote cancer. We’ll discuss that another time.
So grandma was on to something.
However, lipid peroxidation is an ancient process, and our body has evolved a variety of mechanisms to cope with it. “Toxicity of Lipid Oxidation Products Consumed in the Diet” (Shaich, 2020) is an excellent review of them.
But over the 19th, 20th, and 21st centuries our consumption of seed oils containing fats likely to go rancid has increased massively, to levels never before seen (Blasbalg, 2011; Drewnowski, 1997; Lee, 2022). Have these ancient defenses been able to keep up?
Do We Absorb Rancid Fats?
Fats, including oxidized fats, are primarily absorbed from the intestine via packaging into chylomicrons, which are then transported through the lymph system into the general blood circulation, thus bypassing the liver.
In a pair of experiments using human subjects, non-oxidized and oxidized corn oil were fed to normal and diabetic individuals. Levels of oxidized fats were then measured in the chylomicrons in circulation after eating (postprandial, the high peroxide oil).
“Thus, the content of oxidized lipid in the diet determined the levels of oxidized lipid in postprandial chylomicrons.” (Staprãns, 1994)
In this study they used two measures of oxidation, TBARS and conjugated dienes.
A later study by the same group looking at type 2 diabetics found a similar result.
“In summary, the present study demonstrates that after an oxidized lipid–containing meal, in poorly controlled type 2 diabetic patients, the levels of oxidized lipids in the postprandial serum chylomicrons were increased.” (Staprãns, 1999)
Topologically speaking, the digestive tract is ‘outside’ of the body—think of a doughnut, with the hole representing the digestive tract. HNE has been shown to be associated with disease processes in the digestive tract from dental disease (Pradeep, 2013) to the colon (Pierre, 2006), even though it’s technically not yet been absorbed into the body.
HNE, Specifically
Oxidized Ω-6 PUFA products such as HNE are thought to play a “paramount” role in colon cancer, specifically (Cai, 2012).
But are these products absorbed into or created in the rest of the body? (Pierre, 2003; 2006) looked at the question, finding that heme iron (the iron in meat, mostly red meat), which is a potent catalyst of oxidation of PUFAs, causes PUFAs to oxidize into, among other things, HNE. Noting that HNE is detoxified in the body by being converted into DHN-MA (1,4-Dihydroxynonane Mercapturic Acid), which is excreted into the urine (Schaich, 2020), these authors first fed rats, then humans, diets high in heme iron, which rapidly and significantly increased urinary excretion of DHN-MA (Pierre, 2006).
“The effect of diet was very fast because the DHN-MA value increased by a 50-fold factor after only 24 hours on the blood sausage diet. A 5- to 9-fold increase was also seen in rats given a beef meat diet. In human volunteers, the 2-fold DHN-MA increase on the high-heme diet was very significant but much less striking than in rats.” (Pierre, 2006)
The humans were fed a much lower amount of heme iron, and the controls were also fed some, unlike the rat controls which had none, which likely explains much of the dramatic difference between rats and humans.
The authors noted that heme iron alone did not cause the effects seen:
“Diets high … in oxidation-resistant fats, may prevent the possible cancer-promoting effect of red meat…. No difference was seen between rats given… olive oil control diets and rats given the no-[heme] control diet...” (Pierre, 2003)
In a further study, using the same methods, (Keller, 2020) showed that HNE is indeed produced in the intestinal tract:
And that it is absorbed into the body (shown here using carbon-radiolabeled HNE), and further showed that it damages proteins both in the intestinal tract and throughout the organism, by forming HNE-protein adducts.
“Those compounds are involved in the pathogenesis of numerous chronic diseases linked to inflammation processes.” (Pierre, 2006)
DNA Damage
Proteins are not the only molecules at risk of damage from PUFA oxidation products. (Chung, 2003) notes that HNE is implicated in the mutation of the human p53 tumor-suppressor gene in colon (and liver) cancer.
In a “carefully controlled dietary study”, (Nair, 1997) examined the effects of Ω-6 PUFA on various aspects of blood chemistry, primarily the effect on lipids. However, that wasn’t all that they recorded. They also looked at DNA damage:
“Etheno adducts [of DNA] have been proven to be highly miscoding lesions in mammalian cells and are thought to initiate the carcinogenic process through specific point mutations by the known or suspected human carcinogens vinyl chloride or urethane, respectively.” (Nair, 1997)
These authors found a massive sex-dependent increase in these indicators of DNA damage. On average, women had a 40-fold higher rate of damage on the higher-Ω-6 diet, excluding one outlier, the level in the remaining women was from 45- to 76-fold higher.
“Therefore, our results clearly indicate that the DNA adduction was due to consumption of a high Ω-6 PUFA diet, which resulted in elevated levels of polyunsaturated membrane lipids, implying increased lipid peroxidation, although commonly used markers for lipid peroxidation status in serum, such as MA, TBARS, and conjugated dienes, failed to reveal differences between the MUFA and PUPA diet groups.” (Nair, 1997)
While these authors didn’t directly measure HNE, they note that HNE is capable of producing this damage.
Among the other “enals produced by lipid peroxidation” are other toxic Ω-6 metabolites (Chung, 2003).
Given the many toxic effects of HNE, it’s an excellent question if giving humans HNE directly would by ‘ethical’, much like cigarette smoking or barefoot running is discouraged. I’m not aware of any study that has tried giving HNE to humans orally to see what happens. Generally overtly toxic substances are not administered to humans experimentally.
Summary
“The missing link for me is this: Are there studies showing that increasing dietary HNE consumption increases HNE levels in our bodies?”
So here’s the evidence I’ve found to answer this question.
Rancid Fat Experiments
“Oxidized Lipids in the Diet Are a Source of Oxidized Lipid in Chylomicrons of Human Serum.” (Staprãns, 1994)
“Effect of Oxidized Lipids in the Diet on Oxidized Lipid Levels in Postprandial Serum Chylomicrons of Diabetic Patients.” (Staprãns, 1999)
Dental/Systemic Health Correlations
“4-Hydroxy-2-Nonenal, an Oxidative Stress Marker in Crevicular Fluid and Serum in Type 2 Diabetes with Chronic Periodontitis” (Pradeep, 2013)
HNE Production Induced Experimentally
“New Marker of Colon Cancer Risk Associated with Heme Intake: 1,4-Dihydroxynonane Mercapturic Acid.” (Pierre, 2006)
DNA Damage Induced Experimentally
“High Dietary Omega-6 Polyunsaturated Fatty Acids Drastically Increase the Formation of Etheno-DNA Base Adducts in White Blood Cells of Female Subjects” (Nair, 1997)
I think it’s a definite “Yes”, with the fifth experiment above being the clearest, as it actually tracks an HNE metabolite from HNE produced in the gut.
References
Blasbalg, T. L., Hibbeln, J. R., Ramsden, C. E., Majchrzak, S. F., & Rawlings, R. R. (2011). Changes in Consumption of Omega-3 and Omega-6 Fatty Acids in the United States During the 20th Century. The American Journal of Clinical Nutrition, 93(5), 950–962. https://doi.org/10.3945/ajcn.110.006643
Cai, F., Dupertuis, Y. M., & Pichard, C. (2012). Role of Polyunsaturated Fatty Acids and Lipid Peroxidation on Colorectal Cancer Risk and Treatments. Current Opinion in Clinical Nutrition & Metabolic Care, 15(2), 99. https://doi.org/10.1097/MCO.0b013e32834feab4
Chung, F.-L., Pan, J., Choudhury, S., Roy, R., Hu, W., & Tang, M. (2003). Formation of Trans-4-Hydroxy-2-Nonenal- and Other Enal-Derived Cyclic DNA Adducts from Ω-3 and Ω-6 Polyunsaturated Fatty Acids and Their Roles in DNA Repair and Human P53 Gene Mutation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 531(1), 25–36. https://doi.org/10.1016/j.mrfmmm.2003.07.001
Committee on Food Research, Mrak, E. M., Stewart, G. F., & Gelman, G. (1945). Committee on Food Research Conference on Deterioration of Fats and Oils. United States Army Quartermaster Corps.
Drewnowski, A., & Popkin, B. M. (1997). The Nutrition Transition: New Trends in the Global Diet. Nutrition Reviews, 55(2), 31–43. https://doi.org/10.1111/j.1753-4887.1997.tb01593.x
Grootveld, M., Rodado, V. R., & Silwood, C. J. L. (2014). Detection, Monitoring, and Deleterious Health Effects of Lipid Oxidation Products Generated in Culinary Oils During Thermal Stressing Episodes. INFORM Magazine, 25(10), 614–624.
Keller, J., Chevolleau, S., Noguer-Meireles, M.-H., Pujos-Guillot, E., Delosière, M., Chantelauze, C., Joly, C., Blas-y-Estrada, F., Jouanin, I., Durand, D., Pierre, F., Debrauwer, L., Theodorou, V., & Guéraud, F. (2020). Heme-Iron-Induced Production of 4-Hydroxynonenal in Intestinal Lumen May Have Extra-Intestinal Consequences through Protein-Adduct Formation. Antioxidants, 9(12), Article 12. https://doi.org/10.3390/antiox9121293
Le Gresley, A., Ampem, G., De Mars, S., Grootveld, M., & Naughton, D. P. (2021). “Real-World” Evaluation of Lipid Oxidation Products and Trace Metals in French Fries From Two Chain Fast-Food Restaurants. Frontiers in Nutrition, 8. https://doi.org/10.3389/fnut.2021.620952
Lee, J. H., Duster, M., Roberts, T., & Devinsky, O. (2022). United States Dietary Trends Since 1800: Lack of Association Between Saturated Fatty Acid Consumption and Non-communicable Diseases. Frontiers in Nutrition, 8. https://doi.org/10.3389/fnut.2021.748847
Liao, H., Zhu, M., & Chen, Y. (2020). 4-Hydroxy-2-Nonenal in Food Products: A Review of the Toxicity, Occurrence, Mitigation Strategies and Analysis Methods. Trends in Food Science & Technology, 96, 188–198. https://doi.org/10.1016/j.tifs.2019.12.011
Nair, J., Vaca, C. E., Velic, I., Mutanen, M., Valsta, L. M., & Bartsch, H. (1997). High Dietary Omega-6 Polyunsaturated Fatty Acids Drastically Increase the Formation of Etheno-DNA Base Adducts in White Blood Cells of Female Subjects. Cancer Epidemiology and Prevention Biomarkers, 6(8), 597–601. https://cebp.aacrjournals.org/content/6/8/597
Pierre, F., Taché, S., Petit, C. R., Van Der Meer, R., & Corpet, D. E. (2003). Meat and Cancer: Haemoglobin and Haemin in a Low-Calcium Diet Promote Colorectal Carcinogenesis at the Aberrant Crypt Stage in Rats. Carcinogenesis, 24(10), 1683–1690. https://doi.org/10.1093/carcin/bgg130
Pierre, F., Peiro, G., Taché, S., Cross, A. J., Bingham, S. A., Gasc, N., Gottardi, G., Corpet, D. E., & Guéraud, F. (2006). New Marker of Colon Cancer Risk Associated with Heme Intake: 1,4-Dihydroxynonane Mercapturic Acid. Cancer Epidemiology, Biomarkers & Prevention, 15(11), 2274–2279. https://doi.org/10.1158/1055-9965.EPI-06-0085
Pradeep, A. R., Agarwal, E., Bajaj, P., & Rao, N. S. (2013). 4-Hydroxy-2-Nonenal, an Oxidative Stress Marker in Crevicular Fluid and Serum in Type 2 Diabetes with Chronic Periodontitis. Contemporary Clinical Dentistry, 4(3), 281–285. https://doi.org/10.4103/0976-237X.118342
Schaich, K. M. (2020). Toxicity of Lipid Oxidation Products Consumed in the Diet. In Bailey’s Industrial Oil and Fat Products (pp. 1–88). American Cancer Society. https://doi.org/10.1002/047167849X.bio116
Seppanen, C. M., & Csallany, A. S. (2004). Incorporation of the Toxic Aldehyde 4-Hydroxy-2-Trans-Nonenal into Food Fried in Thermally Oxidized Soybean Oil. Journal of the American Oil Chemists’ Society, 81(12), 1137–1141. https://doi.org/10.1007/s11746-004-1031-3
Spiteller, G. (1998). Linoleic Acid Peroxidation—The Dominant Lipid Peroxidation Process in Low Density Lipoprotein—And Its Relationship to Chronic Diseases. Chemistry and Physics of Lipids, 95(2), 105–162. https://doi.org/10.1016/S0009-3084(98)00091-7
Staprãns I, Rapp J H, Pan X M, Kim K Y, & Feingold K R. (1994). Oxidized Lipids in the Diet Are a Source of Oxidized Lipid in Chylomicrons of Human Serum. Arteriosclerosis and Thrombosis: A Journal of Vascular Biology, 14(12), 1900–1905. https://doi.org/10.1161/01.ATV.14.12.1900
Staprãns, I., Hardman, D. A., Pan, X. M., & Feingold, K. R. (1999). Effect of Oxidized Lipids in the Diet on Oxidized Lipid Levels in Postprandial Serum Chylomicrons of Diabetic Patients. Diabetes Care, 22(2), 300–306. https://doi.org/10.2337/diacare.22.2.300


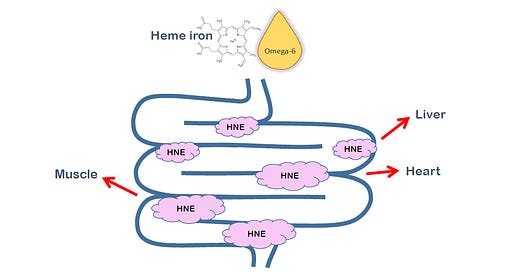










The "intestine is outside the body" part is interesting. If you think about it, that means it's actually "immune" to any PUFA from your own body fat.
So if you stop eating PUFAs today, you'll have all the benefits of a low-PUFA diet pretty much immediately until the point where it enters your bloodstream. From then on out, you're dealing with the memories of PUFA past!
It also makes sense if a lot of HNE is actually produced in there. The heme in the intestines can't oxidize any of the PUFA already in your body fat/blood, right?
I know that beta-oxidation of PUFAs does/can create HNE and other oxidation products in the mitochondria when a fat is burned for fuel. Not sure if there are any other potential oxdiation spots in the body? Is there oxygen around to oxidize?