Quick Study Analysis: Does Eating Fat Cause Aging?
tl;dr: Put down the bowl of rice.
David Sinclair is a professor of genetics at Harvard. He specializes in aging research, and was one of the leading researchers into resveratrol*.
Yesterday he posted this:
331k views, from a guy whose biggest claim to fame is as a failed researcher into resveratrol*. Grr…
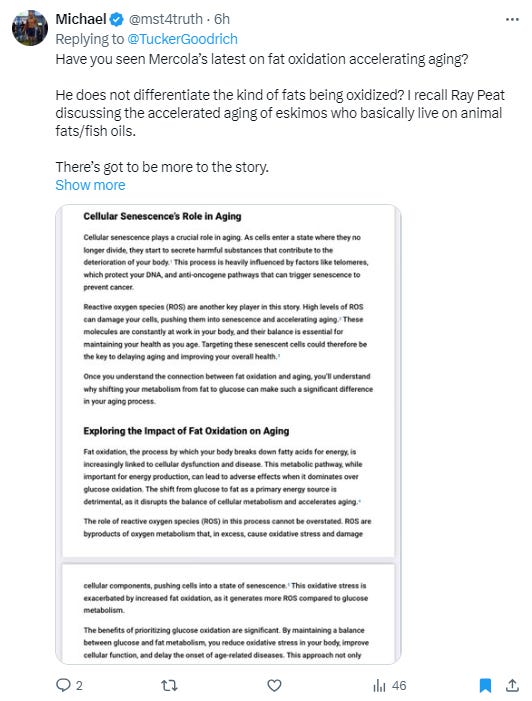
Others are also taking this spin on fat oxidation and spreading it.
The relevant quote in that image is this:
“The shift from glucose to fat as a primary energy source is detrimental, as it disrupts the balance of cellular metabolism and accelerates aging.” (Mercola, 2025)
Is It Plausible?
Is it plausible that using “fat as a primary energy source” or “fuel” is detrimental to health?
If it is, we’re in trouble:
“As you can see from this graph, the available fuel stored in your body—and this is a lean person—is overwhelmingly in the form of fat. Assuming you’re lean, you have about 2,000 kilocalories of carbohydrate stored in your body, and about 40,000 kilocalories of fat.” (Goodrich, 2016)
Fat is the primary energy storage medium in advanced life forms. Now, perhaps we all die because of this fact, but I don’t see how we have any alternative. So it may be plausible, but it’s irrelevant.
However, we have excellent evidence that we were at our healthiest while eating a low-carb diet:
So I don’t think it’s even plausible, and this is without going into the evidence for benefit from a ketogenic diet (at least intermittently) which I think is overwhelming.
What Doesn’t The Study Show?
(Yamauchi, 2024) certainly doesn’t show what Sinclair or Mercola claim it does. It’s an in vitro study of a cell culture. There is no “long term” here, and while they do show that fat induces senescence, they are using caprylic acid (aka octanoic acid/octanoate). It’s found in human breast milk, and in the milk of other mammals as well (it’s named for being found in goat milk). But it’s a trace fat outside of goat milk. Or coconut milk.
At high doses it has lots of useful effects:
“Caprylic acid is an antimicrobial pesticide used as a food contact surface sanitizer in commercial food handling establishments on dairy equipment, food processing equipment, breweries, wineries, and beverage processing plants. It is also used as disinfectant in health care facilities and public places. In addition, caprylic acid is used as an algicide, bactericide, fungicide, and herbicide in nurseries, greenhouses, garden centers, and interiors, and on ornamentation.”
So would you be shocked to find that octanoate (as they call it), provided alone, had some negative effects on these cells?
“Treatment with octanoate or fenofibrate increased mitochondrial ROS levels, decreased mitochondrial membrane potential, and increased the number of cristae and mitochondrial length (fig. S10, E to I).” (Yamauchi, 2024, future references bold and italic)
Fenofibrate is a drug that is used to prevent cardiovascular disease. Its effect is to stimulate fatty acid oxidation (FAO).
“Similar results were obtained with fenofibrate, a lipid-lowering drug that activates FAO through the transcription factor peroxisome proliferator–activated receptor α (PPARα)….”
So both of these compounds are beneficial in humans, not harmful. The dose always makes the poison, of course. You probably shouldn’t drink a bottle of caprylic sanitizer instead of getting it as a supplement—yes, it’s sold as a supplement; and no, I haven’t checked the relative doses.
Further, they’re not even claiming that this increased FAO is a problem.
“This activation may reflect an attempt to repair DNA damage through histone acetylation (44).
Nor do they claim that senescence per se is a problem.
“However, given recent evidence that senescent cells play a role in maintaining tissue structure and function (52), their presence may contribute to the prevention of chronic inflammation in certain tissues.”
As the authors conclude:
“We speculate that prolonged FAO activation, whether due to DNA damage or not, may contribute to the accumulation of senescent cells with age.”
OK, fine, but that’s speculation. Shame on Sinclair and Mercola for not paying better attention.
What Does This Study Tell Us?
“DNA damage has been proposed to be the primary cause of aging, in part because most premature aging syndromes result from mutations in DNA repair genes. Of note, these syndromes and normal aging are accompanied by mitochondrial dysfunction.”
And the clue to the important factor here is from their reference 54:
“FAO is also activated by a high-fat diet and promotes the cancerous transformation of intestinal stem cells (54).”
54 is (Mana, 2021): “High-Fat Diet-Activated Fatty Acid Oxidation Mediates Intestinal Stemness and Tumorigenicity”
And if you’ve been playing this game with me for long enough, the alarm bells should be going off. Yes, the high-fat diet (HFD) used in that paper is the old reliable D12492, last seen here:
If you missed that post, the answer was D12492. (Mana, 2021) provides us with one of the graphical abstracts I love so much:
There’s much evidence that the problematic component of the HFD they are using is the linoleic acid.
But it doesn’t mention linoleic acid or 4-HNE. Neither does (Yamauchi, 2024), for that matter. Sigh.
That’s why you rely on me, I suppose.
(Yamauchi, 2024) uses doxorubicin (dox), a chemotherapy medicine to induce the increase in FAO, and the DNA damage. Dox is a rather nasty drug, but certainly better than dying of cancer, when it works. Part of the effect of doxorubicin (maybe all of it?) is to induce the oxidation of Ω-6 fats in cardiolipin in mitochondria:
“Because oxidative stress caused by dox generates the pro-apoptotic Ω-6 PUFA metabolite 4-hydroxynonenal (4-HNE), we surmised that Ω-6 PUFAs would increase the effectiveness of dox chemotherapy.” (Bose, 2021)
Dox thus depends on the linoleic acid acid in cardiolipin, and is less effective in abnormal cardiolipin without linoleic acid (Aryal, 2016).
I did a long post on linoleic acid in cardiolipin:
And 4-HNE causes the DNA damage that is attributed to dox, “Treatment with doxorubicin, which produces DNA double-strand breaks…”—there may be another pathway also, I can’t rule that out.
“Cytotoxicity, DNA fragmentation and sister-chromatid exchange in Chinese hamster ovary cells exposed to the lipid peroxidation product 4-hydroxynonenal and homologous aldehydes” (Brambilla, 1986)
4-HNE was discovered by Esterbauer, the senior author of that paper, due to its role in cancer.
More recent papers have expanded upon this finding:
“In addition to triggering apoptosis, lipid peroxidation also exerts genotoxicity through mutations as well as single- and double-strand DNA cleavage which can be reversible if normally functioning p53 protein can stop cell cycling to allow DNA repair.” (Bose, 2021)
The increase in FAO is also typical of Ω-6 toxicity, as demonstrated in (Ghosh, 2004):

From this post:
So, while the authors of (Yamauchi, 2024) don’t mention it, pretty much everything they are looking at is (or could be) the result of Ω-6 fat toxicity.
But not fat, generally.
One gets a hint of this in their Fig. 1H, where one can see the unsaturated fat metabolic genes are upregulated, as one would expect if a cardiolipin repair process was stimulated by dox toxicity.

That stimulus is shown by the increased cristae they note. Cardiolipin is the predominant fat in the cristae, it’s primarily composed of unsaturated fats, and is only found in the cristae in the organism.
What About Glucose?
So if you aren’t eating fat, you’re eating glucose for fuel, basically. This could be a lengthy aside, but let’s just note there is another view about the benefits of glucose in longevity.
“A high-sugar diet has been associated with reduced lifespan in organisms ranging from worms to mammals.” (Gusarov, 2017)
As (Ghosh, 2004) makes clear, Ω-6 does much of the damage often attributed to glucose, but I don’t think a high-carb diet is going to extend lifespan.
Other Criticisms
Brenner has made it his mission in life to debunk Sinclair.
With the exception noted above.
It’s a long post, and Sinclair is a professor at the school Nick is attending!
That’s not an accurate enough soundbite. See the conclusion.
So I think it’s fair to say the ‘consensus’ is that we don’t have to worry about fat (in general) causing this particular problem.
Conclusion
So while the pathway that produces the outcomes this study is discussing seems to rely on Ω-6 fats, the study doesn’t “show” much about diet and senescence or aging. What they demonstrate is that the protein of interest (BNIP3) is involved in senescence, and, as others have also shown (Wang, 2025), that Ω-6 are central to this process.
Could excess Ω-6 make it worse? That’s what the research indicates.
“Limiting the intake of Ω-6 PUFAs should aid in cancer prevention by reducing 4-HNE formation...” (Bose, 2021)
Ω-6 is thus a chemotherapy candidate. Toxic, but potentially useful.
So eat your fat, but make it animal-based and low in Ω-6.
* (I have a few old posts on resveratrol that I haven’t transitioned over yet: Here they are. And here’s my summary on resveratrol:
“I'll pass. Call me in 50 years when you finish the long-term studies.”
Glaxo, who bought Sinclair’s company Sirtris, has already ceased research and thrown in the towel (Whalen, 2013), so I guess I’ll never get the chance.
References
Aryal, B., & Rao, V. A. (2016). Deficiency in Cardiolipin Reduces Doxorubicin-Induced Oxidative Stress and Mitochondrial Damage in Human B-Lymphocytes. PLOS ONE, 11(7), e0158376. https://doi.org/10.1371/journal.pone.0158376
Bose, C., Hindle, A., Lee, J., Kopel, J., Tonk, S., Palade, P. T., Singhal, S. S., Awasthi, S., & Singh, S. P. (2021). Anticancer Activity of Ω-6 Fatty Acids through Increased 4-HNE in Breast Cancer Cells. Cancers, 13(24), Article 24. https://doi.org/10.3390/cancers13246377
Brambilla, G., Sciabà, L., Faggin, P., Maura, A., Marinari, U. M., Ferro, M., & Esterbauer, H. (1986). Cytotoxicity, DNA Fragmentation and Sister-Chromatid Exchange in Chinese Hamster Ovary Cells Exposed to the Lipid Peroxidation Product 4-Hydroxynonenal and Homologous Aldehydes. Mutation Research/Genetic Toxicology, 171(2), 169–176. https://doi.org/10.1016/0165-1218(86)90051-0
Ghosh, Sanjoy, Dake Qi, Ding An, Thomas Pulinilkunnil, Ashraf Abrahani, Kuo-Hsing Kuo, Richard B. Wambolt, Michael Allard, Sheila M. Innis, and Brian Rodrigues. Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA. Am J Physiol Heart Circ Physiol 287: H2518 –H2527, 2004. First published July 29, 2004; doi:10.1152/ ajpheart.00480.2004
Goodrich, Tucker D. (2016, February 23). How to Fix Your Metabolism [Blog]. Yelling Stop. https://yelling-stop.blogspot.com/2016/02/how-to-fix-your-metabolism.html
Gusarov, I., Pani, B., Gautier, L., Smolentseva, O., Eremina, S., Shamovsky, I., Katkova-Zhukotskaya, O., Mironov, A., & Nudler, E. (2017). Glycogen Controls Caenorhabditis Elegans Lifespan and Resistance to Oxidative Stress. Nature Communications, 8(1), 15868. https://doi.org/10.1038/ncomms15868
Mana, M. D., Hussey, A. M., Tzouanas, C. N., Imada, S., Barrera Millan, Y., Bahceci, D., Saiz, D. R., Webb, A. T., Lewis, C. A., Carmeliet, P., Mihaylova, M. M., Shalek, A. K., & Yilmaz, Ö. H. (2021). High-Fat Diet-Activated Fatty Acid Oxidation Mediates Intestinal Stemness and Tumorigenicity. Cell Reports, 35(10), 109212. https://doi.org/10.1016/j.celrep.2021.109212
Mercola, J. (2025, January 2). Fat Oxidation—The Hidden Accelerator of Aging and Disease. https://media.mercola.com/ImageServer/Public/2025/January/PDF/fat-oxidation-pdf.pdf
Wang, X., Li, M., Xu, X., Zhao, W., Jin, Y., Li, L., Qin, Z., Sheng, R., & Ni, H. (2025). BNIP3-Mediated Mitophagy Attenuates Hypoxic–Ischemic Brain Damage in Neonatal Rats by Inhibiting Ferroptosis Through P62–Keap1–Nrf2 Pathway Activation to Maintain Iron and Redox Homeostasis. Acta Pharmacologica Sinica, 46(1), 33–51. https://doi.org/10.1038/s41401-024-01365-x
Whalen, J. (2013, March 12). Glaxo to Close Offices of Biotech. Wall Street Journal. http://online.wsj.com/article/SB10001424127887323826704578356633902383560.html
Yamauchi, S., Sugiura, Y., Yamaguchi, J., Zhou, X., Takenaka, S., Odawara, T., Fukaya,-6 S., Fujisawa, T., Naguro, I., Uchiyama, Y., Takahashi, A., & Ichijo, H. (2024). Mitochondrial Fatty Acid Oxidation Drives Senescence. Science Advances, 10(43), eado5887. https://doi.org/10.1126/sciadv.ado588-6 7














Never trust an expert that works at a school.
Is it just my age(70), or is our culture going backwards?
Better to be poor and honest than to be dishonest and a fool.