Quick Study Analysis: What's Worse—Carbs or Seed Oils? Understanding a High-PUFA Diet.
tl;dr: The one study I'm aware of that shows conclusively that excess glucose acts as an accelerant for excess omega-6 fat, but it not nearly so harmful without the omega-6, in vivo.
Introduction
I had this study as my pinned tweet on X (Twitter) for quite a while. (N-6 is short for omega-6 fats). The argument has gotten a little stronger since then, but the study is important to understand, as it best elucidates the mechanism behind the argument concentrated omega-6 fats and not carbohydrates alone are the best candidate for the root cause of the Diseases of Civilization (or chronic diseases).
This is the one study I'm aware of that shows conclusively that excess glucose acts as an accelerant for excess n-6, but it not nearly so harmful without the n-6, in vivo.
"Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA" (Ghosh et al., 2004)
A title only an editor could love, as it buries the lead deeply enough so it could only be found by an archaeologist.
What are they trying to show here?
"Diabetic patients are particularly susceptible to cardiomyopathy independent of vascular disease, and recent evidence implicates cell death as a contributing factor. Given its protective role against apoptosis, we hypothesized that dietary n-6 polyunsaturated fatty acid (PUFA) may well decrease the incidence of this mode of cardiac cell death after diabetes."
"...The majority of studies that have looked at the relations between lipotoxicity and cardiovascular complications of diabetes have utilized lard or other sources of saturated dietary fat rich in palmitic acid (7, 17, 26). However, in humans, increased awareness of obesity and its cardiovascular complications have led to an indiscriminate substitution of atherogenic saturated cooking fats with “heart-friendly” refined vegetable oils, such as sunflower oil, rich in n-6 polyunsaturated fatty acids (PUFA) (42). In several studies, n-6 PUFA conferred protection against arrhythmias (32) and coronary artery disease (12) and, at least in human primary fibroblasts and Leydig cells, prevented apoptosis (4, 31)."
Excellent, seed oils (a more precise term than vegetable oils) are the primary source of such fats, and they're what the Dietary Guidelines suggest we eat to stave off heart disease.
Cardiomyopathy is, basically, a failure of the muscles in the heart. They don't entirely fail, but it's progressive, and also leads to heart failure, which also doesn't indicate total failure, but is obviously not good.
It's not good, but it's also common, and one can find many articles with titles like:
"Heart failure: the cardiovascular epidemic of the 21st century" (Lüscher, 2015)
Or more alarmingly:
"Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure" [3]
Avoiding cardiomyopathy and heart failure is a hot topic.
So what, again, are they doing?
"We hypothesized that, given the role of saturated fatty acids in accelerating cardiac apoptosis after diabetes, switching to an n-6 PUFA-rich diet may well be protective against cell death."
They're trying to test what happens with the "indiscriminate" replacement of saturated animal fats with seed oils in diabetics, using a mouse model.
Since they have a control that is fed a high-carbohydrate diet, both with and without induced diabetes, this also serves as a comparison of the effects of carbohydrates and n-6 PUFA on these animals, although this was not the focus of the paper. As the effects are fairly dramatic, I will highlight those parts.
So here are the steps:
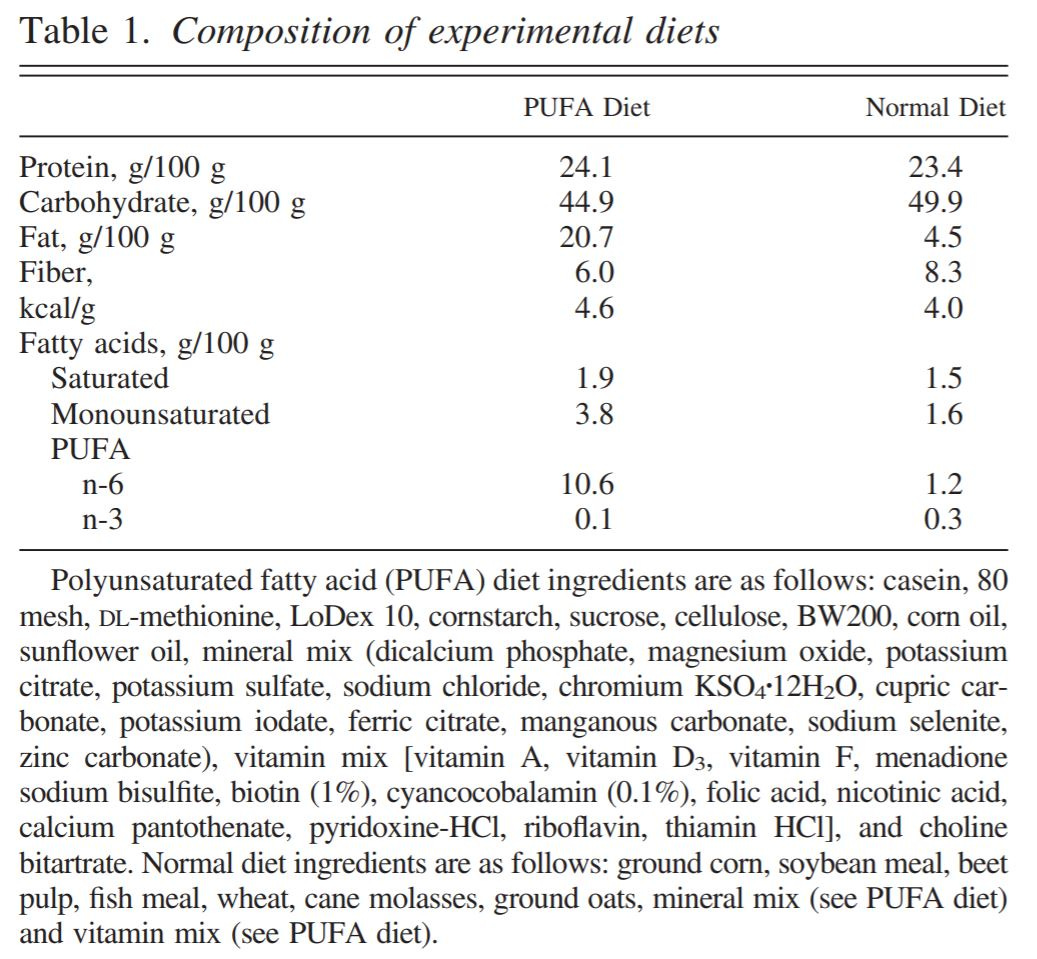
Determine diets. They picked two, and added sunflower to one as a PUFA source. Diet details are good, pretty well-controlled by the generally-abysmal standards of these studies, but they aren't perfectly matched diets like in this study. One has a fair bit more carbs, and the PUFA diet has 2X the monounsaturated fat, 9X the n-6 PUFA, and 1/3 the n-3. Saturated fat was about the same. (See table 1.)
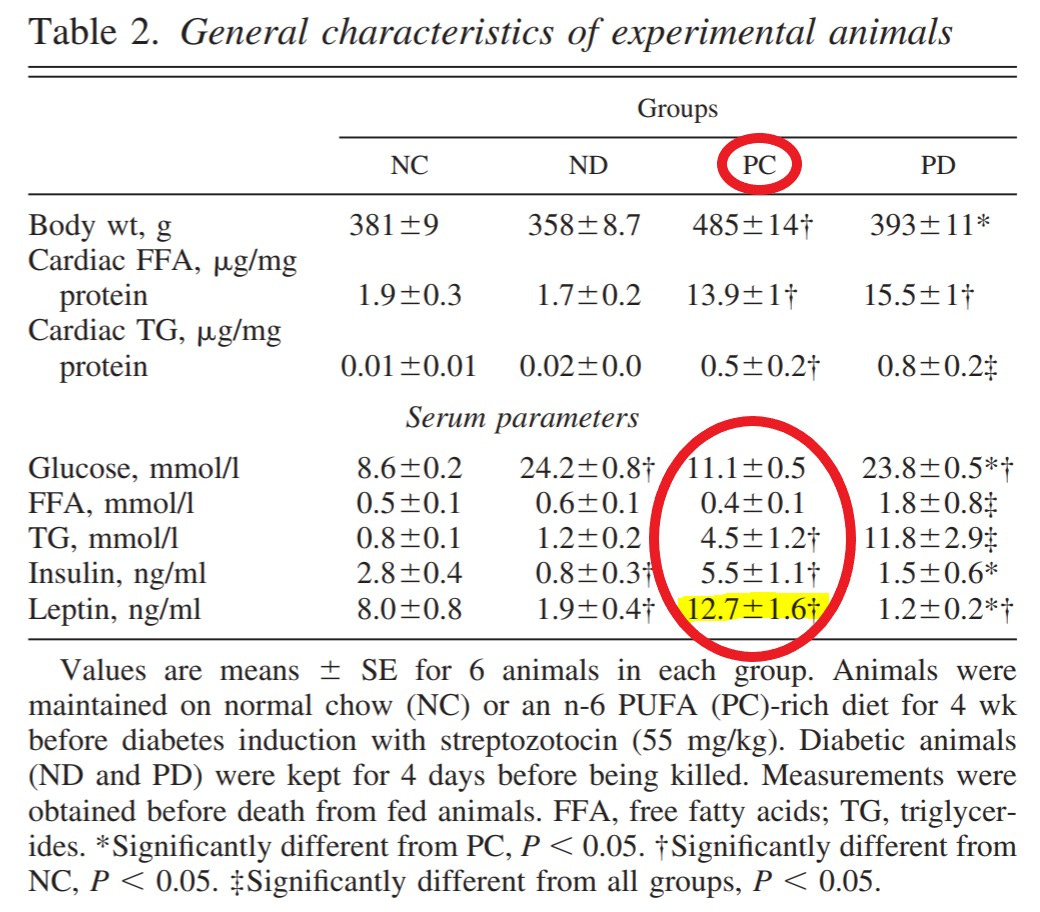
Acquire rats, divide into 4 groups, divided by the traits: Diabetic (D), and Normal (N), and Control (C), and PUFA (P). This gives us Normal Control (NC) Normal Diabetic (ND), and PUFA Control (PC) and PUFA Diabetic (PD) rats. (See table 2.)
Feed all the rats their diets for 4 weeks.
Give the rats in ND and PD groups type 1 diabetes by injecting enough STZ (streptozotocin) to kill half the beta cells in the pancreas.
Let the now type 1 diabetic D-group rats eat for 4 days.
Take serum blood samples, then kill all the rats for analysis.
The Good News
"...We hypothesized that dietary n-6 polyunsaturated fatty acid (PUFA) may well decrease the incidence of this mode of cardiac cell death after diabetes."
Apoptosis is a process of controlled cell death, which allows the body to get rid of damaged cells before say, they become cancerous. While many in the online community get worked up about it, and pursue it through fasting, it's really just a basic house-keeping process, that probably goes on all the time. It's certainly plausible that too much apoptosis could be a bad thing, just as too much of anything could be bad. Clearly having too many heart cells (myocytes) die off could be problematic, and would explain much of heart failure.
"Recently, we demonstrated that feeding a 20% (wt/wt) palm oil diet (rich in palmitic acid) to diabetic rats enhances cardiac apoptosis in vivo..."
They tracked "....caspase-3 activity, the prime effector of cardiomyocyte apoptosis..." and sure enough, apoptosis in the PUFA-fed diabetic rats (PD) was much lower than the non-PUFA fed diabetic rats (ND). Apoptosis in PD was a bit higher than NC or PC, but as a treatment this appeared to be a very good thing. See the red-highlighted bars in Fig. 2 to compare ND to PD. N-6 PUFA for the win!
However, they also checked heart function by another method. They looked at necrosis, and at the heart muscle directly:
"To verify necrosis, serum LDH [lactate dehydrogenase] was estimated using an appropriate kit (Sigma). However, release of LDH does not necessarily imply cardiac necrosis. Thus, to verify cardiac necrosis, sections were evaluated histologically using Masson’s trichrome stain..."
The Bad News
Necrosis is a catastrophic cell death (they use the word "accidental", but it's a bit worse than that) that leads to uncontrolled release of cell contents into the body. Some of these are toxic, and some can induce auto-immune responses, which is why the body prefers to use the orderly process of apoptosis.
In Fig. 3 we can see that LDH skyrockets in the PD rats , those that showed a major benefit from n-6 "control" of apoptosis. It appears that what n-6 is actually doing is shifting the cells from controlled, apoptotic cell death to uncontrolled, necrotic cell death.
"In these hearts, a rise in linoleic acid and depleted cardiac glutathione could explain this “switch” to necrotic cell death."
But they did mention that it's not a sure-fire indicator, and so they had a visual check of heart cells as a backup method.
"Black arrows, severe disruption of contractile apparatus (hypercontracted muscle and thickened fibers); white arrows, focal necrosis (gray discoloration) in... PD hearts (D, 600). Cardiac necrosis was absent in NC (A, 400)..."
Indeed it appears that the n-6 + diabetes rats have suffered a catastrophic onset of heart failure via necrosis. In just four days! N-6 did indeed "protect" them from apoptosis, by shifting to a worse outcome.
More Bad News: Cardiolipin Levels Collapsed, as Did Mitochondria
I've written three long posts on the role and impact of excess n-6 in the mitochondria as affects cardiolipin (CL):
(Transition note: these are still on Blogger, but will move over.)
So I won't bore you here. To summarize, CL is a key molecule in the mitochondria that preferentially takes up n-6, and then breaks down into toxins such as HNE, often destroying the mitochondria, and perhaps the cell, in the process.
Here we have an n-6 intervention, with resultant cell death via necrosis. Was CL in the mitochondria involved, as my posts above would predict?
Luckily these researchers looked at it.
Figure 5 shows the catastrophic effect of n-6 on cardiolipin in both wings to which it is fed, and the much smaller, but still additive, effect of induced diabetes.
"Total cardiolipin decreased almost sixfold after n-6 PUFA feeding, with a significant drop of ATP only in PD hearts, which could have contributed to cardiac necrosis (40)."
As CL is a crucial molecule in the mitochondria, one would expect that such a dramatic reduction in CL content would also have an effect on the mitochondria, since CL is required for ATP production. (ATP is the body's basic energy carrier.) The paper also looks at morphology, the shape of the mitochondria, both normal and n-6-fed.
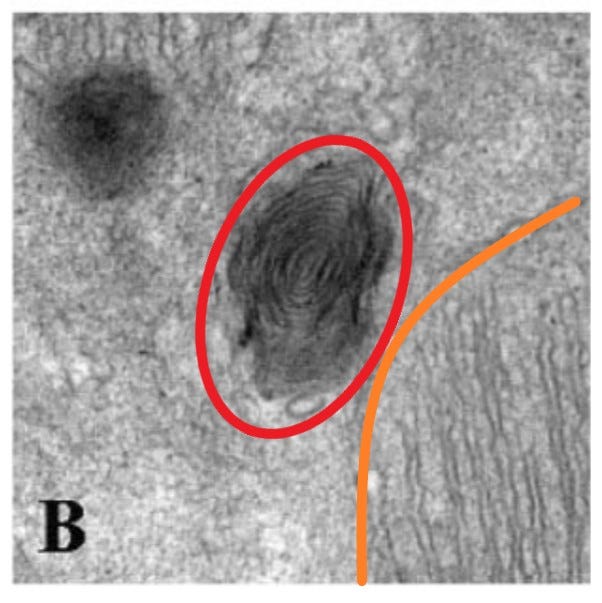
"Figure 5A depicts a mitochondrion with a double membrane and lamellar cristae, which are typical in NC, ND, and PC hearts. A novel observation in this study was abnormal condensed mitochondria, but only in the PD group (Fig. 5B)." [Image A not shown]
Hence n-6 consumption has a catastrophic effect on mitochondria, and hyperglycemia induces even worse pathology.
Bad News Continues: Mitochondrial Function Reduced
The next question is the functional impact of these alterations of CL content and mitochondrial form.
The authors therefore look at the effect of these alterations on fuel use.
And sure enough, they were rather severe. The authors (details in the paper) take the hearts from the dead animals and run fuel through them, creating a controlled environment where they can see precisely what fuel is used and how much.
"To meet the energy demand at high afterload (135 mmHg), all except the PD group increased their glucose oxidation. With regard to fatty acid oxidation, only the NC group was able to increase palmitate oxidation, suggesting that, in the ND, PC, and PD groups, fatty acid oxidation was already operating at its maximum at low afterloads."
The lack of glucose oxidation (red circle, Fig. 6 B) indicates a near-total failure in mitochondrial Complex I, where glucose is burned. The study detailed in my post 3 notes a high production of reactive oxygen species (ROS) in complex I induced by n-6, and the results of this study suggest that n-6 induced ROS may terminally damage complex I and thus the cell's ability to utilize glucose.
"A novel observation in this study was that, analogous to Barth’s syndrome, n-6 PUFA feeding for 4 wk, together with 4 days of hyperglycemia, led to similar changes in some mitochondria. Interestingly, abnormal condensed mitochondria have been recently recognized in skeletal muscle and cardiac atrial neurons from diabetic patients (25)."
This is a notable finding, as excess ROS production and an inability to utilize glucose is a primary feature of Alzheimer's Disease, which is also referred to as Type III Diabetes and for which n-6-induced damage is an oft-observed possible cause.
Mitochondrial Toxins Increased
In my post 2 above I noted that the breakdown of n-6 fat linoleic acid in CL produces a toxin, HNE. As production of some amount of HNE is a normal part of mitochondrial function, there's a detoxification mechanism, part of which is the antioxidant glutathione—inexplicably abbreviated everywhere as GSH. While the authors don't mention HNE directly—the mechanism for CL producing HNE wasn't found until 2012—they do look at GSH. Since GSH levels are often used as a proxy for HNE production, this is a useful measure.
"PUFA feeding was associated with a significant decrease in GSH. Interestingly, diabetes for 4 days in normal chow-fed rat hearts could not decrease cardiac GSH, whereas superimposition of diabetes in PUFA-fed animals led to a further decrease in cardiac GSH levels (Fig. 4A)"
In Fig. 4 A, one sees that GSH is indeed reduced, least in ND rats, most in PD rats. There is additional discussion in the paper about some further indicators, but I'm not going to discuss them as they don't add much to the point made here.
HNE is the leading cause of genetic damage and also induces amyloid-beta plaque development in Alzheimer's, so it's something we want to minimize, and the implication of this data is that n-6 in these amounts overwhelms the body's ability to detoxify it.
This would explain one of the more inexplicable aspects of chronic diseases of civilization, that of mitochondrial dysfunction. Here it is shown how to induce it.
Worst News: Now They Have Heart Failure
The failure in energy production, as the mitochondria are the primary engines of the heart, has consequential effects on heart function. Function decreases, and the ability to respond to increased demands declines dramatically, as the semi-functional mitochondria are no longer able to meet those demands.
"At higher afterloads, glucose and fatty acid oxidation increased in the NC group. In ND and PC hearts, the only increase was that of glucose oxidation, inasmuch as, presumably, fatty acid oxidation was already operating at its maximum. The PD group alone failed to increase its glucose oxidation in response to a higher energy demand, an aspect that could have contributed to the drop in cardiac function."
Additionally, they have some spiffy pictures of the progression of cellular dysfunction from NC to the poor PD rats, who are clearly in a rough spot. I'll spare you those, as I presume by now the gist is clear.
This failure of the capacity to produce energy using fats causes a back-up of fats in the fat cells, as delivery is exceeding demand.
"In our study, n-6 PUFA feeding substantially increased cardiac fatty acid in the PC and PD groups, but only PD hearts demonstrated a considerable increase in lipid droplets."
They've replicated what's described in this paper [4], in the section entitled "Evidence that Human Cardiac Dysfunction Is Associated with Excess Lipid", where lipid droplets are seen in diabetic and/or failing human hearts.
N-6 Induced Obesity
The weight figures from Table 2 fail to tell an important story. The figures given are those when the rats were killed, but the highest weight in the D-groups was at STZ injection. See the chart (mine) to see what a significant increase n-6 caused in the rats' weights prior to STZ, which caused a dramatic decline only in the rats fed n-6.
"Interestingly, in contrast to the ND group (361 ± 9 and 358 ± 9 g before and after STZ, respectively), diabetes in PUFA-fed animals was associated with a profound loss of body weight (450 ± 11 and 393 ± 11 g before and after STZ, respectively). This loss in body weight could not be attributed to any change in food or fluid intake but could be a result of excessive lipolysis and loss of adipose tissue mass with subsequent increases in serum free fatty acids and TG in the PD group."
This is consistent with Alvheim's series of papers starting in 2012 showing that n-6 linoleic acid uniquely induces obesity in rodents (Alvheim et al., 2012).
A Bonus Bad-News Easter Egg: The Control Got Diabetes, Too
"Thus, although promoted as being beneficial, excess n-6 PUFA, with its predisposition to induce obesity, insulin resistance, and ultimately diabetes, could accelerate myocardial abnormalities in diabetic patients... Circulating TG were higher in the PC than in the NC group, and serum insulin was increased in the PC group (likely as a consequence of insulin resistance) compared with the NC group, but there was no overt hyperglycemia in the PC group."
They don't really focus on this, but it's kind of a big deal. They had two variables they were testing, and this left them with four groups, as detailed in Table 2. Therefore they had one "control", NC, but the intervention was supposed to be the PD group. But let's look at the PC group, too. The quote above notes they didn't get "overt" hyperglycemia, but, compared to the full control, their:
Blood glucose +29%
Triglycerides (TG) +462%
Insulin +96%
Leptin* +59%
The D-groups had Type 1 diabetes induced, the PC group got Type 2 diabetes because of the intervention: n-6 induced it!
Back to "overt" hyperglycemia. The PC rats didn't get it because:
"In rodents fed high-fat diets, insulin resistance does not progress to hyperglycemia in the absence of genetic defects (36)."
So these rats seem to have gotten as close to Type 2 diabetes as it's possible for them to have gotten.
Conclusion
"In summary, chronic caloric excess of n-6 PUFA when coupled with acute diabetes of only 4 days precipitated mitochondrial abnormalities, a steep drop in GSH, altered substrate utilization, and myocardial TG deposition. Given that these hearts also demonstrated necrosis and extensive myocardial cell loss, a feature that is predominant only in chronic diabetes (1, 14, 16, 24), our data suggest that this mode of cell death in PUFA-fed diabetic hearts is an important factor in accelerating diabetic cardiomyopathy. Although these effects of n-6 PUFA in the diabetic animal would seem contrary to accepted belief as being beneficial, in countries such as Israel, with high dietary n-6 PUFA consumption, there is an excessive incidence of obesity, insulin resistance, hypertension, and type 2 diabetes (6).... Thus advocating diets rich in n-6 PUFA for diabetic patients could accelerate the impairment of myocardial contractility."
This also is the only study I know of that shows the effects of hyperglycemia to significantly accelerate the negative effects of n-6 PUFA feeding on an organism. The PC and PD groups can be seen as a model for the progression of obesity and insulin/leptin resistance to an advanced form of chronic Type 2 with advanced pancreatic beta-cell death, a condition that is becoming increasingly common in human children.
Combined with my previous post, "Hello, Can We Have Your Liver?": Understanding a High-PUFA Diet, we have evidence of n-6 involvement in two major chronic organ failures, and the hints of causation of diabetes, a disease impacting all organs.
(I'll also note that I've been told this paper was a Trojan horse, that the authors knew very well what the paper was going to show, but to get it published kow-towed to the Temple of Vegetable Oils, to make it look like another cheerleading pro-PUFA study. They feign surprise that this does not seem to be the case, but I think that in this sentence, with it's pejorative "indiscriminate" and scare quotes around "heart-friendly", they let the truth slip out. Luckily the editors and reviewers didn't catch it!
"However, in humans, increased awareness of obesity and its cardiovascular complications have led to an indiscriminate substitution of atherogenic saturated cooking fats with “heart-friendly” refined vegetable oils, such as sunflower oil, rich in n-6 polyunsaturated fatty acids (PUFA) (42)."
Good for them!)
* A hormone that is supposed to control fat accumulation.
References
Alvheim, A. R., Malde, M. K., Osei‐Hyiaman, D. , Hong, Y. H., Pawlosky, R. J., Madsen, L. , Kristiansen, K. , Frøyland, L. and Hibbeln, J. R. (2012), Dietary Linoleic Acid Elevates Endogenous 2‐AG and Anandamide and Induces Obesity. Obesity, 20: 1984-1994. doi:10.1038/oby.2012.38
Ghosh, Sanjoy, Dake Qi, Ding An, Thomas Pulinilkunnil, Ashraf Abrahani, Kuo-Hsing Kuo, Richard B. Wambolt, Michael Allard, Sheila M. Innis, and Brian Rodrigues. Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA. Am J Physiol Heart Circ Physiol 287: H2518 –H2527, 2004. First published July 29, 2004; doi:10.1152/ ajpheart.00480.2004
Goldberg, Ira J. et al. Lipid Metabolism and Toxicity in the Heart, Cell Metabolism, Volume 15 , Issue 6 , 805 - 812
Lavie, Carl J., Alpert, Martin A., Arena, Ross, Mehra, Mandeep R., Milani, Richard V., Ventura, Hector O.. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure
JACC: Heart Failure Apr 2013, 1 (2) 93-102; DOI:10.1016/j.jchf.2013.01.006
Lüscher, Thomas F.. Heart failure: the cardiovascular epidemic of the 21st century, European Heart Journal, Volume 36, Issue 7, 14 February 2015, Pages 395–397, https://doi.org/10.1093/eurheartj/ehv004

















Fascinating. I knew about the cardiolipin problem but not the apoptosis replaced by uncontrolled necrosis. I dropped seed oils many years ago, at that point with evolution in mind; we had not evolved to have so much kinky lipids in our cells so it was bound to have negative effects. Then I picked up on 4-HNE protein binding. The other positive of using saturated fats is no smelly, stuck up frying pans - that tells you something.
Tuck, this is excellent analysis, as I would expect. Interestingly, just yesterday or the day before, I came across a familiar Instagram Health Influencer, interviewing another "thinker" who seemed to be making the case that, "contrary to what all the loud voices tell you, seed oils are nearly as bad as saturated fats" or words to that effect. I thought to myself, "what a maroon" and almost kept it moving. Upon further review, what he seemed to be saying is that if one overfeeds, overfeeding on saturated fats is worse than overfeeding on seed oils. (Frankly, I was so disgusted, I kind of stopped listening closely!) I remain shocked that there has not been almost-complete adoption of what seems obvious to me. Something along the lines of "Seed oils are bad, mkay?" Still not there yet. What am I missing?