Does Our Body Treat Seed Oils Like Toxins? (The Fat Curve, Part 2)
tl;dr: A follow-up to a 2018 post. Just because they look like they're healthy it doesn't mean they are.
Introduction
If you drink alcohol, your body tries to get rid of it. It’s toxic, and while it has calories, the body doesn’t have any way to store it or process it without suffering from the toxic effects.
So the body prioritizes getting rid of it, through oxidation, mostly.
And to do that it down-regulates using everything else for fuel. In other words, the body preferentially oxidizes alcohol. This doesn’t mean it likes alcohol as a fuel—we won’t get into social effects or addiction—but it must get rid of it (Lieber, 1985).
Glucose and other carbohydrates are similar. Excess glucose in the bloodstream is toxic, but unlike alcohol we have uses for carbohydrates. We can store them, and produce them on demand when needed. Did you know sperm use fructose, the toxin, for fuel? But too much carbohydrate causes the body to start treating it like a toxin: it stores it (unlike alcohol) in a safe form (glycogen), it converts it to things that are better for storage, and less harmful (saturated fats), and it oxidizes it, turning it into waste heat. I discuss this process in this post (and see more below):
What about fats? In this post below (from 2018, orginally) I looked at what happens to your body (if you are a mouse) when you eat a high-linoleic acid (LA, the primary fat from seed oils) diet.
Interestingly, eating 8% of calories as LA makes you fat, but 34% doesn’t.
So does that mean that the other fats are the problem, and we’re all just not eating enough seed oils, as the American Heart Association (more another day) and the Federal Government would have us believe? Well, not quite.
Does High LA Protect From Obesity?
I discussed in Part 1 of The Fat Curve that there is an interplay between different levels of Ω-6 fats, low and high levels are non-obesogenic, but intermediate levels, especially with other fats, are.
The other day, Peter from Hyperlipid posted this:
Similar to the post in “Can We Have Your Liver”, except a lot more corn oil, but with a crucial difference in this paper.
We were having a discussion about causes of obesity. Peter had said, “They came close to uncoupling levels of LA.” His position is that an inherent trait of LA is that it induces “uncoupling”, or the oxidation of fats without production of the ATP that is usually the point of mitochondrial oxidation, and that this prevents the obesity that is caused by an excess of calories, due to the absence of insulin resistance (a good thing in that scenario). I’m probably over-simplifying this, but do read this post which I think is a good explanation of his point. (Dobromylskyj, 2020)
No-Obesity LA depends on IBAT
So in this paper (Matsumura, 2018) they fed mice either a chow diet, or a chow + corn oil diet. Neither group of mice got obese.
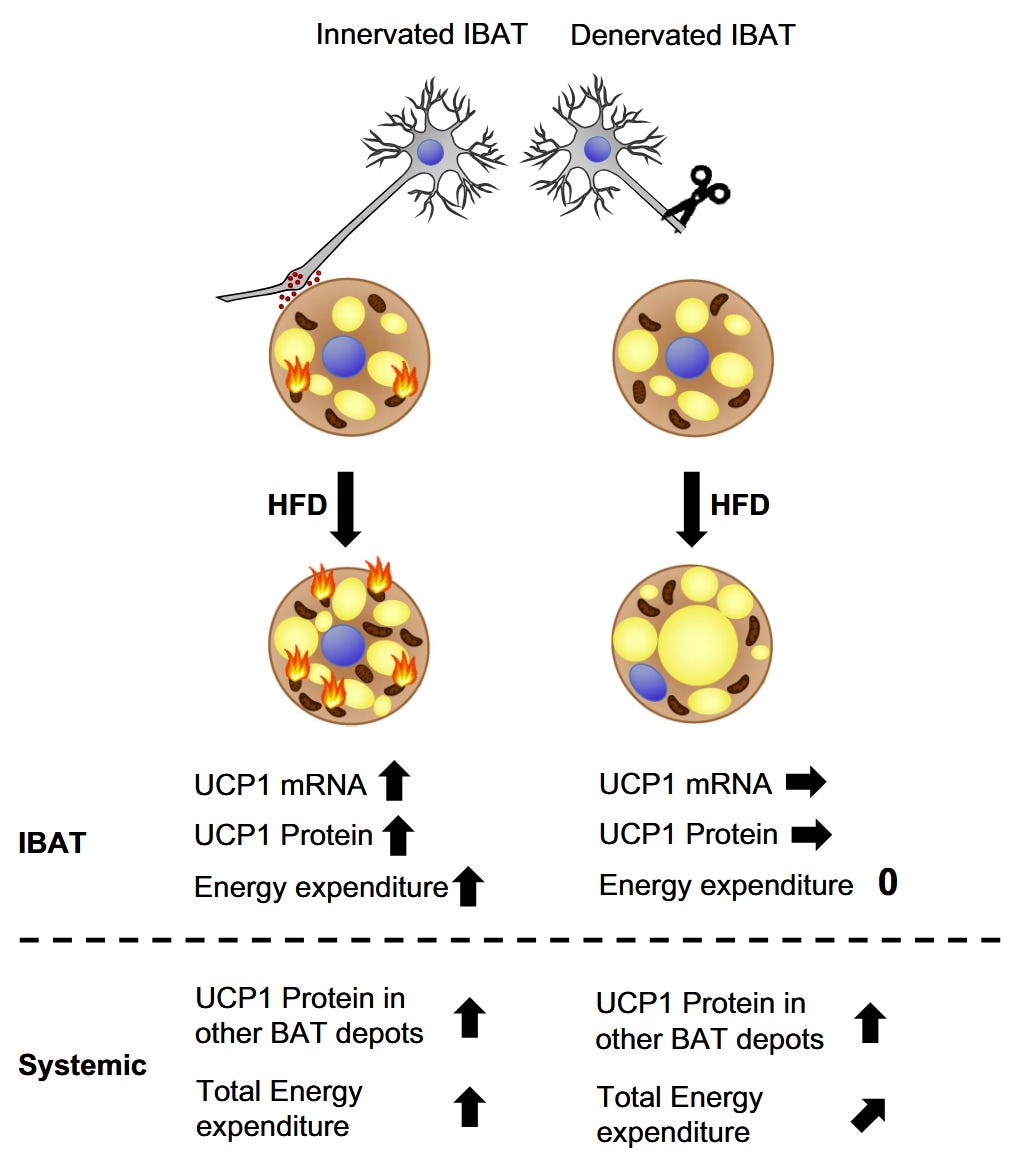
IBAT (interscapular BAT) is the brown adipose tissue that lies along the back in mice and in humans. Its purpose, mainly, is to keep us warm when the temperature drops. In humans, many have very small amounts of this fat, since we live in largely temperature-controlled environments.
The authors severed the nerve to the IBAT (BSNTX). Or they surgically removed the IBAT. (“Sham” means they cut the animal but didn’t sever the nerve, it’s a placebo surgery, essentially.)
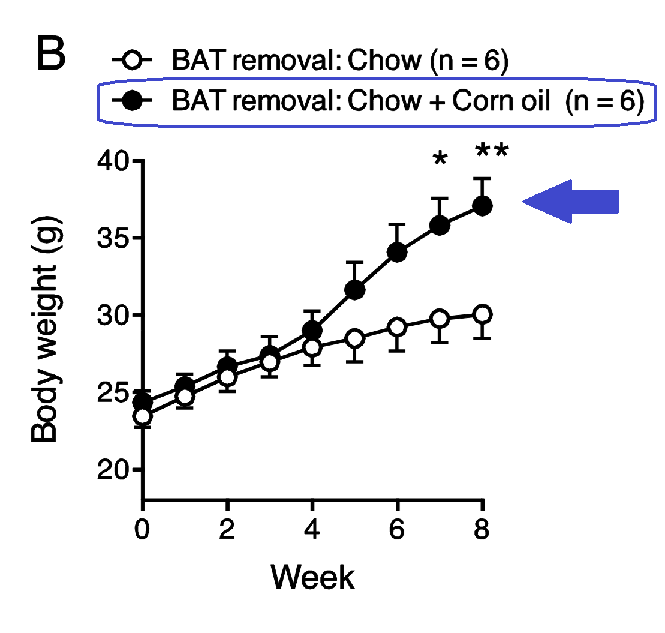
Severing the nerve to IBAT caused weight gain, but IBAT removal surgery was even more effective at restoring the obesogenic effect of corn oil.
The red arrow points to about 25g bodyweight, the upper blue arrow (fig. A) to ~32g, and the lower blue (fig. B) to ~37g.
Oxygen consumption also declined after both BSNTX and IBAT removal surgery, indicating a decreased oxidation of fat.
So the mice’s disposal pathway was removed. They were no longer able to burn off the excess LA, and so it went into adipose tissue. Whatever baseline IBAT function remained after severing the nerve was completely removed by removing IBAT.
Sadly they didn’t measure the weight of the IBAT in the chow + corn oil compared to the chow group.
Peter hypothesizes in these two posts (Dobromylskyj, 2021, April 9) and (Dobromylskyj, 2021, April 10) discussing (Matsumura, 2018), and in the second states:
“Simply looking at the experimental data the situation appears to be that including linoleic acid at levels between somewhere just under 10% up to somewhere in the high 20%s will facilitate excess insulin action and excess lipid storage. As we increase the supply of linoleic acid through the 30%s and in to the 40% region of calories then uncoupling becomes the primary molecular solution to a progressively more intractable problem.” (Dobromylskyj, 2021, April 10)
The fat curve, in other words. However, the implication throughout these posts is that this increased uncoupling is happening in all mitochondria. If that was true, then the surgeries might have little effect. IBAT is a small percentage of body weight, but it seems to concentrate the uncoupling reaction to excess LA. This is not a molecular response to LA in the mitochondria, it’s a systemic response. The organism is attempting to protect itself from excess LA, as it would from excess glucose or from alcohol.
Low-LA Lard Is Not Protective
In (Fischer, 2019), they perform some similar experiments, but get drastically different results. They use a slightly different mouse, C57BL/6J vs /6N (Fisher-Wellman, 2016), but they have a similar-enough response to high-fat feeding such that it shouldn’t make a difference (the /6N mouse is more susceptible to the ill effects of ‘high fat’ feeding).
But they feed them lard instead of corn oil, with a lot less linoleic acid (6.2 % of energy). The surgery has no effect on these mice, they get just as fat with or without a functioning IBAT nerve.

They offer no speculation as to why this difference might exist.
“Thus, it must be concluded that the presence of an intact nervous system supply to the tissue is at least absolutely essential for the recruitment process associated with [diet-induced thermogenesis] (Fig. 9). A very recent study with another feeding model arrived at a similar conclusion (51).” (Fischer, 2019)
Ref. 51, “another feeding model”, is (Matsumura, 2018), using corn oil.
One would think these scientists would be the least bit curious about why these other researchers found a totally different result, but nope. They likely discovered this at the end of their writing and didn’t want to re-do the whole thing. Sigh.
“Thus, in sham-operated mice, chronic exposure to a high-fat diet led to a clear increase in daily energy expenditure (Fig. 3C). In the denervated mice, this increase was halved (Figs. 3D and 9 and Table 1).” (Fischer, 2019)
So they at least they both saw that effect. I should also note that the modified mice gained more weight in the IBAT than the non-modified mice, suggesting that fat was delivered to IBAT, but then did not receive the signal to combust.
Corn Oil Increases IBAT Mitochondrial Mass
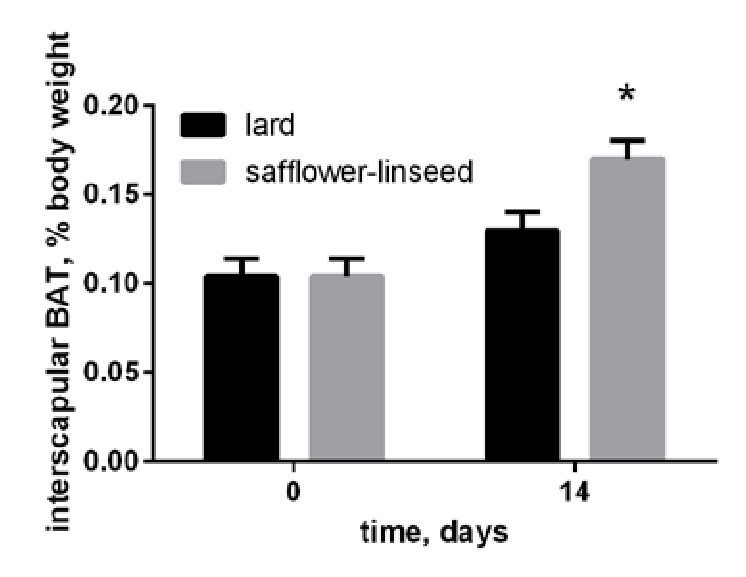
One of the things that I noted in the “Hello, Can We Have Your Liver?” post was that the brown adipose tissue of the lean mice expanded quite dramatically in the seed-oil fed mice:
Others have noted that a high-Ω-6 ketogenic diet also induce increases in the mitochondrial proteins (bigger mitochondria, not more) in BAT, and that these mitochondria were malformed (Srivastava, 2013).

Another study (Mercer, 1987) used a different mouse, the C57BL/6J ob/ob obesity-prone mouse (Thurlby, 1979). We won’t get into why, but these mice will get fat almost no matter what you feed them—a trait true to some extent of all the mice discussed, since mice that won’t get fat are not used in obesity research (Collins, 2004) !
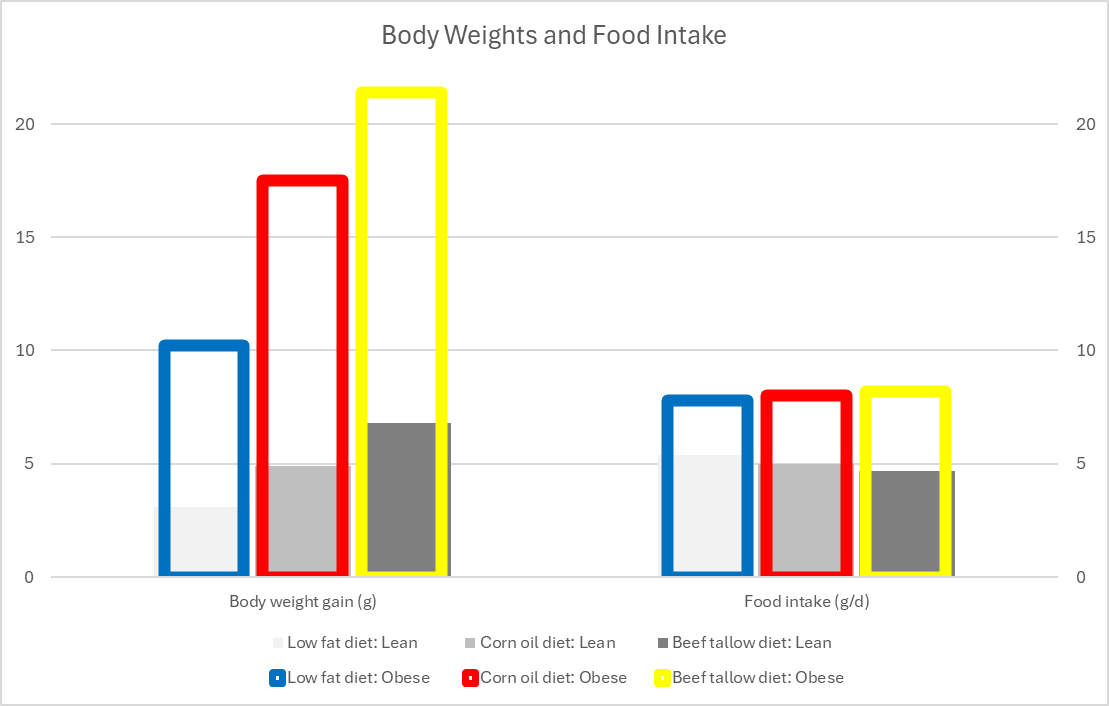
They fed one group corn oil and the other beef tallow, so two drastically different levels of LA:
“Corn oil contains predominantly polyunsaturated fatty acids and has a linoleic (C18:2) acid content of approximately 60% (with 26% [oleic (C18:1)]). Beef tallow is rich in saturated and monounsaturated fatty acids and has the following fatty acid composition: 41% [oleic] C18:1, 19% [stearic] C18:0 and 30% [palmitic] C16:0 + [palmitoleic] C16:1 (21).” (Mercer, 1987)
Tallow typically has a low level of LA, ~2%. The tallow-fed mice gained more weight in either the base C57BL/6 line (Lean) or the ob/ob line (Obese). Food intake per day was similar between the two lines of mice, with the obese mice eating substantially more than the lean.
Nevertheless, despite eating the slightly more energy (ME), the corn oil mice did not deposit that intake into adipose tissue to the same extent as those fed tallow (31% vs. 56%, respectively).
One of the hallmarks of the obese mice discussed here is a decrease in their metabolic rate at lower temperatures. As mentioned, one of the major roles of IBAT is thermoregulation.
“At thermoneutrality (33°[C]) the resting metabolic rate of adult ob/ob and lean mice, when expressed in absolute terms (ml oxygen/h per animal), is the same, whereas at lower temperatures the metabolic rate of the obese is 20% below that of the lean (Trayhurn & James, 1978). This indicates that at normal environmental temperatures (20-25°[C]) ob/ob mice expend less energy than lean animals on the thermoregulatory thermogenesis needed to maintain body temperature.” (Thurlby, 1979)
In these animals, this is reflected in a higher fat content of the IBAT, and a lower mitochondrial content.
“Cytochrome oxidase activity was lower in the ob/ob mice fed the low fat diet or the beef tallow diet than in lean littermates fed the same diet. However, the obese mice fed the com oil diet exhibited a twofold increase in activity relative to the other groups of obese animals. Lean mice fed the corn oil diet also had significantly higher cytochrome oxidase activity than lean mice fed either of the other two diets. Thus, in both genotypes, feeding a corn oil diet resulted in a substantially greater mitochondrial mass and oxidative capacity of brown adipose tissue.” (Mercer, 1987)
They summarize:
“A diet rich in polyunsaturated fatty acids appears to result in preferential stimulation of the thermogenic activity of brown adipose tissue, particularly in the ob/ob mouse.” (Mercer, 1987)
The Standard, D12492
So I found a study that uses my favorite (hah) diet, D12492. D12492 has around 17% of energy as linoleic acid (Liu, 2013). In this case, IBAT function is suppressed by deleting (knocking out, KO) the REV-ERB nuclear receptors in a part of the brain called the dorsal vagal complex, “the DVC REV-ERBa/b double knockout (DVC RDKO):” (Woodie, 2023)
“The central roles of REV-ERBs are primarily behavioral such that loss of expression in the whole body or brain induces disturbances in sleep, memory, and social behaviors. However, in specific groups of neurons or brain areas REV-ERBs control centrally mediated metabolic functions and it is unknown whether the DVC is one of these areas” (Woodie, 2023)
I’m going to avoid going down this particular rabbit hole at this point, let’s just suffice to say that deleting these hormone receptors has the same effect of severing the nerve to the IBAT, function is reduced, but only in response to D12492.

There is no increase in body weight in the KO mice in the “normal chow diet”. Food intake is similar to the non-KO mice. However, there is a massive increase in bodyweight over the already-obesogenic D12492 diet.

Oxygen intake is also dramatically reduced.
The REV-ERB nuclear receptors are thyroid/steroid hormone receptors involved in circadian rhythm regulation (Lazar, 1989), thus closing the circle with my post:
Apparently the thyroid-system hormones are also involved in the disposal of excess PUFA, as is adrenalin. Thyroid hormone upregulates uncoupling protein 1 (UCP1—the protein that allows brown mitochondria to produce the waste heat that is the primary source of thermogenesis) and mitochondrial biogenesis (increased quantity of mitochondria) in humans (Lee, 2012).
There are a number of other interesting things I’d like to get into, but this is long enough.
Summary
Peter summarizes his second post thus:
“TLDR: If resisting insulin is rendered impossible due to very, very high PUFA diets then mitochondria resort to uncoupling.” (Dobromylskyj, 2021, April 9)
The primary location of uncoupling is the IBAT mitochondria. UCP1 is primarily, almost entirely, found in IBAT mitochondria. Mitochondria in other organs have from low to nonexistent levels of the other uncoupling proteins—the liver has none (Shabalina, 2014)—and there is some debate over what is the function of the other UCP proteins, exactly. UCP1 KO causes a short-term loss of thermoregulation—UCP1 KO mouse pups will die in a day if not kept in a warm environment—but given time to adapt, UCP1 KO mice can adapt to cold temperatures despite the lack of an obvious pathway for doing so (Liu, 2003).
High dietary linoleic acid induces compensatory upregulation of mitochondrial function (Abuirmeileh, 1980) and in IBAT mitochondria, mass (Mercer, 1987). Removing IBAT or impairing its function worsens linoleic-acid induced obesity (Mercer, 1987; Fischer, 2019; Woodie, 2023). But absence of functional IBAT mitochondria does not stimulate any compensatory adaptation to linoleic-induced obesity in the rest of the body, apparently.
UCP1 KO mice have no increased risk of obesity, and in fact have lower body weights when kept in a cold environment, due to higher fat oxidation rates (Matthias, 1999; Liu, 2003). Humans with UCP1 loss-of-function mutations also do not seem to suffer excessively from obesity (Chen, 2015; Nicoletti, 2016).
Deficits in uncoupling do not seem to stimulate the rest of the body’s mitochondria to take up this burden of uncoupling: likely they can’t through this mechanism (Ježek, 2015; Nicoletti, 2016).
However, there is another source of uncoupling in mitochondria: fatty acids. Specifically fatty acids in the mitochondrial membrane . This provides a brake on reactive oxygen species (ROS, like H2O2) production by unsaturated fats in the mitochondria.
“A comparison of the efficacy of various fatty acids on hydrogen peroxide generation in respiring mitochondria is shown in Fig. 5. Oleic and linoleic acids had an effect similar to that elicited by arachidonic acid with both succinate and pyruvate + malate as substrate. Palmitic acid was much less effective. It caused, in fact, H2O2 generation at a rate that was 60% of the maximal potential rate at site I and only 17% of that at site II.”
It’s unlikely that the primary role of the UCP1 protein is control of ROS. IBAT produces much more ROS than, say muscle mitochondria, and it does so if it is uncoupled or not (Schönfeld, 2012).
Why Does Excess Linoleic Acid Increase Brown Fat?
(Matsumura, 2018) notes:
“We also observed that small amounts of fat emulsion (Intralipid) applied to the tongue stimulated sympathetic nerve activity innervated to IBAT in urethane anesthetized rats (unpublished data).”
Rodents, unlike humans, are adapted to a diet high in seeds, and thus high in linoleic acid. If linoleic acid is inherently problematic, then it shouldn’t surprise us to find that there is a disposal pathway, like for alcohol or excess glucose. That a high-LA fat like Intralipid can stimulate IBAT activity just from taste suggests that this is a fundamental pathway.
Leukotoxin
In humans and rodents linoleic acid can be metabolized to various bioactive metabolites. One of them is known as leukotoxin (12,13-diHOME), which I discuss at length in this post:
Aside from the effects described in that post in reaction to inflammation or infection, leukotoxin stimulates the growth of IBAT, and the “browning” of normal adipose tissue, making it more like brown adipose tissue, able to assist in thermoregulation.
Leukotoxin is produced by cells after exposure to cold (Abe, 2022; Lynes, 2017) or exercise (Stanford, 2018).
“Murine experiments show that BAT is the tissue source of exercise-induced increases in circulating 12,13-diHOME, and that this lipokine increases fatty acid uptake in skeletal muscle in vivo.” (Stanford, 2018)
Might production of leukotoxin be the reason that linoleic acid increases the mitochondrial mass of IBAT? It’s conceivable.
BAT alone seems to be protective against metabolic derangement, one can speculate this might be due to a role in humans in lowering linoleic acid levels.
Is Linoleic Acid an Anti-Obesity Food?
Aside from the liver damage described in this post:
A high-linoleic diet in the form of D12492 also seems to directly damage BAT.
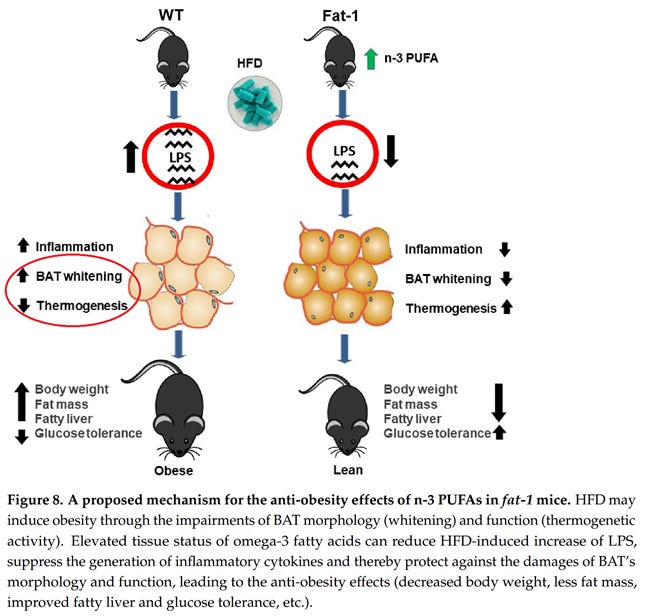
So I think the answer is: probably not. Excess linoleic acid induces “morphological and functional impairments of brown fat”, as the title of (Hao, 2022) explains. So even if one might benefit from a short-term increase in consumption, the evidence that long-term over-consumption is harmful is overwhelming.
Peter and I both agree on that.
References
Abe, I., Oguri, Y., Verkerke, A. R. P., Monteiro, L. B., Knuth, C. M., Auger, C., Qiu, Y., Westcott, G. P., Cinti, S., Shinoda, K., Jeschke, M. G., & Kajimura, S. (2022). Lipolysis-Derived Linoleic Acid Drives Beige Fat Progenitor Cell Proliferation. Developmental Cell, 57(23), 2623-2637.e8. https://doi.org/10.1016/j.devcel.2022.11.007
Abuirmeileh, N. M., & Elson, C. E. (1980). The Influence of Linoleic Acid Intake on Electron Transport System Components. Lipids, 15(11), 925–931. https://doi.org/10.1007/BF02534416
Chen, Y., Wang, X., Shen, Z., Fan, P., Liu, R., Liu, Y., Ren, R., Ma, L., & Bai, H. (2015). Effect of the Beta-3 Adrenergic Receptor Trp64Arg and Uncoupling Protein 1-3826 A>G Genotypes on Lipid and Apolipoprotein Levels in Overweight/Obese and Non-Obese Chinese Subjects. Lipids in Health and Disease, 14, 34. https://doi.org/10.1186/s12944-015-0029-y
Cocco, T., Di, M., Papa, P., & Lorusso, M. (1999). Arachidonic Acid Interaction with the Mitochondrial Electron Transport Chain Promotes Reactive Oxygen Species Generation. Free Radical Biology and Medicine, 27(1), 51–59. https://doi.org/10.1016/S0891-5849(99)00034-9
Collins, S., Martin, T. L., Surwit, R. S., & Robidoux, J. (2004). Genetic Vulnerability to Diet-Induced Obesity in the C57BL/6J Mouse: Physiological and Molecular Characteristics. Physiology & Behavior, 81(2), 243–248. https://doi.org/10.1016/j.physbeh.2004.02.006
Di Paola, M., & Lorusso, M. (2006). Interaction of Free Fatty Acids with Mitochondria: Coupling, Uncoupling and Permeability Transition. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1757(9), 1330–1337. https://doi.org/10.1016/j.bbabio.2006.03.024
Dobromylskyj, P. (2020, September 9). Protons (60) 4-Hydroxy-2-Nonenal [Blog]. Hyperlipid. https://high-fat-nutrition.blogspot.com/2020/09/protons-60-4-hydroxy-2-nonenal.html
Dobromylskyj, P. (2021, April 9). Hyperlipid: The ginger paradox (4) [Blog]. Hyperlipid. https://high-fat-nutrition.blogspot.com/2021/04/the-ginger-paradox-4.html
Dobromylskyj, P. (2021, April 10). Hyperlipid: The ginger paradox (5) and some speculation [Blog]. Hyperlipid. https://high-fat-nutrition.blogspot.com/2021/04/the-ginger-paradox-5-and-some.html
Dobromylskyj, P. (2025, April 1). @TuckerGoodrich @SFNostalgia @BenBikmanPhD @Blagosklonny @garytaubes Of course. ~35%E corn oil -> ~17% LA. Try 84%E as corn oil -> slim. Https://pubmed.ncbi.nlm.nih.gov/30192428/ https://high-fat-nutrition.blogspot.com/2021/04/the-ginger-paradox-4.html https://t.co/W07OB9ASho [Tweet]. Twitter. https://x.com/Peter_InNorfolk/status/1906917931620970822
Fischer, A. W., Schlein, C., Cannon, B., Heeren, J., & Nedergaard, J. (2019). Intact Innervation Is Essential for Diet-Induced Recruitment of Brown Adipose Tissue. American Journal of Physiology. Endocrinology and Metabolism, 316(3), E487–E503. https://doi.org/10.1152/ajpendo.00443.2018
Fisher-Wellman, K. H., Ryan, T. E., Smith, C. D., Gilliam, L. A. A., Lin, C.-T., Reese, L. R., Torres, M. J., & Neufer, P. D. (2016). A Direct Comparison of Metabolic Responses to High-Fat Diet in C57BL/6J and C57BL/6NJ Mice. Diabetes, 65(11), 3249–3261. https://doi.org/10.2337/db16-0291
Hao, L., Nie, Y.-H., Chen, C.-Y., Li, X.-Y., Kaliannan, K., & Kang, J. X. (2022). Omega-3 Polyunsaturated Fatty Acids Protect against High-Fat Diet-Induced Morphological and Functional Impairments of Brown Fat in Transgenic Fat-1 Mice. International Journal of Molecular Sciences, 23(19), Article 19. https://doi.org/10.3390/ijms231911903
Ježek, J., Dlasková, A., Zelenka, J., Jabůrek, M., & Ježek, P. (2015). H2O2-Activated Mitochondrial Phospholipase iPLA2γ Prevents Lipotoxic Oxidative Stress in Synergy with UCP2, Amplifies Signaling via G-Protein–Coupled Receptor GPR40, and Regulates Insulin Secretion in Pancreatic β-Cells. Antioxidants & Redox Signaling, 23(12), 958–972. https://doi.org/10.1089/ars.2014.6195
Kulterer, O. C., Niederstaetter, L., Herz, C. T., Haug, A. R., Bileck, A., Pils, D., Kautzky-Willer, A., Gerner, C., & Kiefer, F. W. (n.d.). The Presence of Active Brown Adipose Tissue Determines Cold-Induced Energy Expenditure and Oxylipin Profiles in Humans. The Journal of Clinical Endocrinology & Metabolism. https://doi.org/10.1210/clinem/dgaa183
Lazar, M. A., Hodin, R. A., Darling, D. S., & Chin, W. W. (1989). A Novel Member of the Thyroid/Steroid Hormone Receptor Family Is Encoded by the Opposite Strand of the Rat C-ERBA Alpha Transcriptional Unit. Molecular and Cellular Biology, 9(3), 1128–1136.
Lecker, J. (n.d.). Mouse Diet, High Fat, Fat Calories (60%), Soft Pellets ( F3282, control F4031). Bio-Serv. Retrieved October 4, 2021, from https://www.bio-serv.com/product/HFPellets.html
Lee, J.-Y., Takahashi, N., Yasubuchi, M., Kim, Y.-I., Hashizaki, H., Kim, M.-J., Sakamoto, T., Goto, T., & Kawada, T. (2012). Triiodothyronine Induces UCP-1 Expression and Mitochondrial Biogenesis in Human Adipocytes. American Journal of Physiology. Cell Physiology, 302(2), C463-472. https://doi.org/10.1152/ajpcell.00010.2011
Lieber, C. S. (1985). Alcohol and the Liver: Metabolism of Ethanol, Metabolic Effects and Pathogenesis of Injury. Acta Medica Scandinavica, 218(S703), 11–55. https://doi.org/10.1111/j.0954-6820.1985.tb08903.x
Liu, X., Rossmeisl, M., McClaine, J., & Kozak, L. P. (2003). Paradoxical Resistance to Diet-Induced Obesity in UCP1-Deficient Mice. The Journal of Clinical Investigation, 111(3), 399–407. https://doi.org/10.1172/JCI15737
Liu, T.-W. (2013). Changes in Fatty Acids Profiles After Three Weeks of High-Fat Diet Feeding in Obesity-Prone Rats [Master’s Dissertation, University of Missouri—Columbia]. https://doi.org/10.32469/10355/44001
Lynes, M. D., Leiria, L. O., Lundh, M., Bartelt, A., Shamsi, F., Huang, T. L., Takahashi, H., Hirshman, M. F., Schlein, C., Lee, A., Baer, L. A., May, F. J., Gao, F., Narain, N. R., Chen, E. Y., Kiebish, M. A., Cypess, A. M., Blüher, M., Goodyear, L. J., … Tseng, Y.-H. (2017). The Cold-Induced Lipokine 12,13-Dihome Promotes Fatty Acid Transport into Brown Adipose Tissue. Nature Medicine, 23(5), 631–637. https://doi.org/10.1038/nm.4297
Matsumura, S., Ishikawa, F., Sasaki, T., Odanaka, M., Manio, M. C. C., Fushiki, T., & Inoue, K. (2018). Voluntary Corn Oil Ingestion Increases Energy Expenditure and Interscapular UCP1 Expression Through the Sympathetic Nerve in C57BL/6 Mice. Molecular Nutrition & Food Research, 62(22), e1800241. https://doi.org/10.1002/mnfr.201800241
Matthias, A., Jacobsson, A., Cannon, B., & Nedergaard, J. (1999). The Bioenergetics of Brown Fat Mitochondria from UCP1-Ablated Mice. UCP1 Is Not Involved in Fatty Acid-Induced De-Energization (“uncoupling”). The Journal of Biological Chemistry, 274(40), 28150–28160. https://doi.org/10.1074/jbc.274.40.28150
Mercer, S. W., & Trayhurn, P. (1987). Effect of High Fat Diets on Energy Balance and Thermogenesis in Brown Adipose Tissue of Lean and Genetically Obese Ob/Ob Mice. The Journal of Nutrition, 117(12), 2147–2153. https://doi.org/10.1093/jn/117.12.2147
Nicoletti, C. F., de Oliveira, A. P. R. P., Brochado, M. J. F., de Oliveira, B. P., Pinhel, M. A. de S., Marchini, J. S., dos Santos, J. E., Salgado Junior, W., Silva Junior, W. A., & Nonino, C. B. (2016). UCP1 -3826 A>G Polymorphism Affects Weight, Fat Mass, and Risk of Type 2 Diabetes Mellitus in Grade III Obese Patients. Nutrition, 32(1), 83–87. https://doi.org/10.1016/j.nut.2015.07.016
Schönfeld, P., & Wojtczak, L. (2012). Brown Adipose Tissue Mitochondria Oxidizing Fatty Acids Generate High Levels of Reactive Oxygen Species Irrespective of the Uncoupling Protein-1 Activity State. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1817(3), 410–418. https://doi.org/10.1016/j.bbabio.2011.12.009
Shabalina, I. G., Vrbacký, M., Pecinová, A., Kalinovich, A. V., Drahota, Z., Houštěk, J., Mráček, T., Cannon, B., & Nedergaard, J. (2014). ROS Production in Brown Adipose Tissue Mitochondria: The Question of UCP1-Dependence. Biochimica Et Biophysica Acta, 1837(12), 2017–2030. https://doi.org/10.1016/j.bbabio.2014.04.005
Srivastava, S., Baxa, U., Niu, G., Chen, X., & Veech, R. L. (2013). A Ketogenic Diet Increases Brown Adipose Tissue Mitochondrial Proteins and UCP1 Levels in Mice. IUBMB Life, 65(1), 10.1002/iub.1102. https://doi.org/10.1002/iub.1102
Stanford, K. I., Lynes, M. D., Takahashi, H., Baer, L. A., Arts, P. J., May, F. J., Lehnig, A. C., Middelbeek, R. J. W., Richard, J. J., So, K., Chen, E. Y., Gao, F., Narain, N. R., Distefano, G., Shettigar, V. K., Hirshman, M. F., Ziolo, M. T., Kiebish, M. A., Tseng, Y.-H., … Goodyear, L. J. (2018). 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metabolism, 27(5), 1111-1120.e3. https://doi.org/10.1016/j.cmet.2018.03.020
Thurlby, P. L., & Trayhurn, P. (1979). The Role of Thermoregulatory Thermogenesis in the Development of Obesity in Genetically-Obese (ob/Ob) Mice Pair-Fed with Lean Siblings. British Journal of Nutrition, 42(3), 377–385. https://doi.org/10.1079/BJN19790127
Woodie, L. N., Melink, L. C., Alberto, A. J., Burrows, M., Fortin, S. M., Chan, C. C., Hayes, M. R., & Lazar, M. A. (2023). Hindbrain REV-ERB Nuclear Receptors Regulate Sensitivity to Diet-Induced Obesity and Brown Adipose Tissue Pathophysiology. Molecular Metabolism, 79, 101861. https://doi.org/10.1016/j.molmet.2023.101861

















If mice are adapted to a high-LA diet because they eat seeds, wouldn't we expect them to be immune to the obesogenic effects of LA e.g. the 8% Alvheim studies? Or is this just an effect of breeding crazy strains?
"High dietary linoleic acid induces compensatory upregulation of mitochondrial function... Excess linoleic acid induces “morphological and functional impairments of brown fat”. So even if one might benefit from a short-term increase in consumption, the evidence that long-term over-consumption is harmful is overwhelming."
Clearly, long-term omega-3/6 balance modulates longevity. Typically researchers recommend omega-3 supplementation to achieve omega-3/6 balance as noted in this AI Overview that appears when one does an 'arachidonic acid intake endocannabinoid tone longevity' web search: "Arachidonic acid (AA), an omega-6 fatty acid, is a precursor for endocannabinoids like anandamide (AEA) and 2-arachidonoylglycerol (2-AG). These endocannabinoids play a role in various physiological processes, including appetite, energy metabolism, and neuroplasticity. While AA is essential, excessive intake can lead to increased endocannabinoid tone and potentially contribute to conditions like obesity and inflammation. Balancing omega-6 and omega-3 fatty acids, with a focus on increasing omega-3 intake (EPA and DHA), can help modulate endocannabinoid levels and improve health outcomes." https://pubmed.ncbi.nlm.nih.gov/34067450/
(web search - ectopic adipose tissue arachidonic acid longevity) AI Overview: "Ectopic adipose tissue, or fat accumulation in areas other than traditional fat stores, and the presence of high levels of arachidonic acid (AA), a fatty acid, are both linked to inflammation and a reduction in lifespan. Ectopic fat contributes to metabolic dysfunction, insulin resistance, and chronic low-grade inflammation, while AA is a precursor for pro-inflammatory molecules. These factors can lead to a shorter lifespan and the development of age-related diseases."
(web search - Ectopic arachidonic acid impaired fatty acid oxidation) AI Overview: "Ectopic arachidonic acid accumulation, meaning excess arachidonic acid in locations outside its normal cellular context, can impair fatty acid oxidation, a process by which the body breaks down fats for energy. This impairment can lead to a buildup of other fatty acids, contributing to cellular dysfunction and potentially inflammation."
(web search - ectopic adipose tissue linoleic acid longevity) AI Overview: "While linoleic acid (LA), an omega-6 fatty acid, is a crucial part of a healthy diet and plays a role in various bodily functions, excessive intake, especially in the context of ectopic fat accumulation and aging, may have negative implications for longevity. Linoleic acid is a major component of adipose tissue, and changes in its levels, particularly due to increased dietary intake, can impact metabolic health and potentially accelerate aging."
This narrative suggests that the most favorable health outcome is achieved when intake of polyunsaturated fats does not exceed physiological requirements by a significant margin. "The degree of fatty acid unsaturation of mitochondrial membrane lipids has been found to be one of those biochemical parameters that are most strongly correlated with longevity, when different species of mammals and birds are compared, with a low degree of fatty unsaturation being correlated with less lipid peroxidation and a longer normal life-span." https://lipidworld.biomedcentral.com/articles/10.1186/1476-511X-9-37