Does Linoleic Acid Induce Obesity? Part 2: Roux-en-Y Gastric Bypass and 4-Hydroxynonenal
Examining more of the mechanisms and human outcome data for the relationship between seed oils and obesity.
Roux-en-Y Gastric Bypass (RYGB)
After Roux-en-Y bypass surgery, people show "a marked reduction of the hedonic drive to consume palatable food and beneficial changes in dietary habits." RYGB excludes from food contact the primary part of the small intestine that mediates food reward.
— Stephan Guyenet, PhD (@whsource) November 29, 2018
That study is (Ullrich et al., 2013), which is interesting in its observations, but, as they note, “The mechanism underlying the reduction in hedonic hunger after RYGB surgery cannot be derived from our data… Whatever the underlying mechanisms might be, it is reasonable to assume that the reduction in hedonic hunger largely contributes to the success of RYGB surgery.”
Emphasis mine. As discussed in Part 1, much of the activity in the endocannabinoid hyperphagia signaling appears to take place in the small intestine, although mediated through the brain (Higuchi et al., 2012). (DiPatrizio et al., 2011) found, “Notably, severing the vagus nerve below the diaphragm abolished the ability of fat to initiate small-intestinal endocannabinoid production…”, and (Avalos et al., 2020) found that “Cannabinoid CB1 Receptors in the Intestinal Epithelium Are Required”, for LA-induced obesity.
Together these effects constitute serious evidence for the Food Reward Hypothesis, which I consider to be pretty incontrovertible at this point. They also, however, demonstrate that n-6 linoleic acid appears to play a central role in this process, in animals and in humans.
So do we have an idea what the mechanism is behind the “success” of RYGB?
I put “success” in scare quotes for RYGB because while it often—not always—reduces obesity, it also has a number of very negative effects on the patients who submit to the procedure. Rimonabant was withdrawn from the market due to similar, yet milder effects on patients.
“Roux-en-Y gastric bypass (RYGB) was the most commonly performed procedure (58.9%). The post-bariatric suicide event rate was 2.7/1000 patients… while the suicide/self-harm attempt event rate was 17/1000 patients… The self-harm/suicide attempt risk was higher after bariatric surgery within the same population with OR of 1.9… and compared to matched control subjects, OR 3.8...” (Castaneda et al., 2019)
In English, you’re 4x more likely to try to hurt or kill yourself after having this surgery. So, I wouldn’t recommend this procedure, unless you have no other alternatives.
A Rodent Model of RYGB
In 2004 a surgeon at Syracuse University developed a rat model of RYGB, so as to better understand the effects this procedure was having on human patients.
“Of the numerous operations developed during the past decades, the Roux-en-Y gastric bypass (RYGB) is considered among the most successful and safe operations following which morbidly obese patients usually lose an average of 49% to 65% of their initial body weight within 2 to 5 years. Besides the robust weight loss, the surgical procedure ameliorates obesity-related diseases (initially identified as syndrome X and now commonly referred to as “metabolic syndrome”), particularly diabetes and hyperlipidemia.” (Meguid et al., 2004)
Yes, in surgery you come up with the animal model after you’ve experimented on humans (Faria, 2017; W O Griffen, 1977), the reverse of the practice in every other area of medicine.
Given what we’ve learned so far, we can probably guess what they were using to induce obesity in these rats: “…consisting of 8% corn oil…”. The rest was carbohydrates. That didn’t seem to do the trick well enough, so:
“…Ten days before the operation, rats on the high-energy diet were also fed the highly palatable liquid supplement Boost-Plus… providing 1.52 kcal/ ml, of which 16% of the metabolizable energy content was protein, 34.0% was fat, and 50% was carbohydrate, to enhance their weight gain.” (Meguid et al., 2004)
Boost Plus contains: “Water, glucose syrup, sugar, vegetable oil (canola, high oleic sunflower, corn), milk protein concentrate,” and some minor ingredients (BOOST Plus®, 2021).
Boost Plus is marketed as a weight-gain solution, for humans, and is a competitor to Ensure, (Abbott Laboratories, Inc., 2020; Ensure vs. Boost, 2020), discussed in Part 1 as being as obesogenic as the lab diets used to fatten up rodents (DiPatrizio et al., 2011).
Now what is most interesting about this surgery is that it almost immediately cures type 2 diabetes (T2DM).
“Weight loss is not the reason why GB controls diabetes mellitus. Instead, bypassing the foregut and reducing food intake produce the profound long-term alterations in glucose metabolism and insulin action. These findings suggest that our current paradigms of type 2 diabetes mellitus deserve review. The critical lesion may lie in abnormal signals from the gut.” (Hickey et al., 1998)
Emphasis mine. And:
“The changes in insulin resistance seen after gastric bypass, which are responsible for the resolution or improvement of type 2 diabetes occur within 6 days of the surgery, before any appreciable weight loss has occurred. This finding has implications for our understanding of the mechanism of insulin resistance in severely obese patients and is consistent with a humoral mechanism emanating from the GI tract.” (Wickremesekera et al., 2005)
“Humoral mechanism” means one involving the immune system. Not a bad supposition, at all.
Hormones Matter in Obesity
(Rubino et al., 2004) at the same time were looking into the hormonal effects of RYGB in humans, and found that many hormones were affected, although they did not propose a mechanism for which one might have the effect.
Further work noticed a fundamental difference in hormone activation between those with surgery and those merely eating less.
“Consistent with previous reports, postoperative ghrelin levels did not show the rise that would be predicted by diet-induced weight loss. This may partly explain the reduced appetite following gastric bypass. It is uncertain what causes this abrogation of the anticipated compensatory rise in ghrelin levels.” (Borg et al., 2006)
Although they note:
“The variable degree of vagal [nerve] disconnection of the gastric pouch after RYGB may also play a role in some patients.” (Borg et al., 2006)
In Part 1, looking at the mechanisms of the endocannabinoid system (ECS), we discussed the vagal/vagus nerve:
“Notably, severing the vagus nerve below the diaphragm abolished the ability of fat to initiate small-intestinal endocannabinoid production (Fig. S2), which indicates that neural communication from the brain to the gut via the vagus nerve is necessary to drive this biochemical reaction. These findings suggest that cephalic signals elicited by sham feeding of fat, but not other nutrients, selectively mobilize endocannabinoids in the upper gut through a mechanism that is mediated by efferent vagal fibers.” (DiPatrizio et al., 2011)
Continuing the work, senior author Meguid et al. note:
“In contrast to our catabolic RYGB rat model, in animal models of cancer cachexia, in cachectic human patients with cardiac heart failure and in anorexia nervosa, plasma ghrelin levels are increased. This rise has been interpreted as an attempt to compensate for the catabolic–anabolic imbalance of cancer cachexia. In our model such a compensatory mechanism does not occur, given that most of the ghrelin-producing cells are bypassed because they are located primarily in the residual defunctionalized stomach.
“The decreased amount of ingested food passes from the pouch to the distal small bowel, bypassing the critical neuroendocrine organs of the distal stomach, duodenum, pancreas, liver, biliary system and proximal small bowel. This surgical procedure is similar to that performed in obese patients.” (Guijarro et al., 2006)
Over the following two years they continue to drill down to the mechanism by which a 30-year-old surgical procedure works, they finally make a connection:
“Also, our data suggest an important role of the vagus in the outcome of RYGB surgery, whose contribution to the weight loss in this procedure has not been adequately explored.”
“Could the RYGB procedure, therefore, also affect the endocannabinoid system?... The CB1R antagonist SR-141716 [rimonabant] induces long-lasting weight loss, independent in part of food intake, by deceasing fatty acid synthesis (56) and increasing metabolic activities (38, 47) and/or energy expenditure as suggested by the increase in basal oxygen consumption in ob/ob mice after chronic intraperitoneal administration (47)…” (Guijarro et al., 2007)
Emphasis mine. (We’ll explore exactly how this could happen later, in our discussion of HNE.)
It’s Not Just Any Hormones…
Meguid’s group continues:
“Using this model, we tested our hypothesis that down-regulation of the peripheral endocannabinoid system along with alterations in mitochondrial function and hormones favoring catabolic processes contribute to sustained weight loss after RYGB surgery by counterbalancing the compensatory changes in energy intake and expenditure that occur with time. Thus, results from our study will provide surgeons with insight into the mechanism of successful sustained weight loss after RYGB in morbidly obese patients and could potentially lead to CB1 receptor antagonist becoming a postoperative adjunct drug for RYGB patients who fail to maintain reduced weight loss and to prevent weight regain.”
So if your RYGB surgery fails, which it does 20% of the time (Guijarro et al., 2007), they’ll just give you rimonabant, since it has the same effect. How did this turn out?
“In conclusion, RYGB induces a coordinated down-regulation of the endogenous cannabinoid system and enhanced mitochondrial function, leading to normalization of the dysregulated physiological processes associated with obesity.” (Guijarro et al., 2008)
Unfortunately, this is just an association. While successful RYGB surgery caused a change in ECS function, this doesn’t establish that the permanent reduction in weight status was the result of this ECS change, although given that a change in ECS function via rimonabant induces a similar change in weight status, inferring causation is quite reasonable, and is what these authors do.
A different group looked at the effects of RYGB on food reward (Shin et al., 2011). As covered ad nauseum in Part 1, the ECS is central to food reward, as was amply demonstrated. These authors seemed unaware of that literature, or of the literature cited above, as the don’t mentioned the ECS or the RYGB literature from Meguid’s group at all (was Louisiana really that far from upstate New York in 2011?).
“Although it appears plausible that changes in gut hormone secretion cause these beneficial effects after RYGB, the specific mechanisms involved are completely unknown at this point.” (Shin et al., 2011)
Kindly, however, they elected to use the ‘cookie-dough diet’ (D12492) (Research Diets, Inc., 2006b), and Ensure (Abbott Laboratories, Inc., 2020), which we discussed above and in Part 1, to fatten these rats.
In fact, their examination of food reward was quite similar to that of other researchers, assuming that these effects are mediated by the ECS:
“In OP rats, ‘liking’ of the highest sucrose concentration was significantly blunted by RYGB.” (Shin et al., 2011)
Compared to:
“Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors” (Arnone et al., 1997)
“Specifically related to the ECS it has been shown that CB1 activation increases the response to sweet taste (Yoshida et al., 2009)." (Witkamp, 2018)
“[RYGB patients] consumed distinctly less fatty sweets such as chocolate (3.2±0.2 vs. 2.7±0.2; P00.048), less cake, biscuits, and cookies (3.4±0.2 vs. 3.0±0.2; P00.09), and also tended to consume less fruit juice/soft drinks (3.8±0.3 vs. 3.2±0.3; P 00.08) than preoperatively.” (Ullrich et al., 2013)
And others (Duarte et al., 2004; Koch, 2001; Simiand et al., 1998).
However, Shin et al. did not explore what the mechanism was beyond the fact of the surgery, however their title was quite sound, “Roux-en-Y Gastric Bypass Surgery Changes Food Reward in Rats” (Shin et al., 2011).
We’ve now gone through enough of the literature on RYGB and its effects on eating to conclude that (Ullrich et al., 2013) should have had an idea of what was reducing ‘hedonic’ eating. However, they did not cite the line of research done by Meguid’s group or the endocannabinoid research.
Other reviews of the topic similarly do not mention the ECS line of research (Hansen et al., 2016; Harvey et al., 2010), but work continued.
One important finding of (DiPatrizio et al., 2011) deserves notice:
“Sham feeding of fat fails to affect endocannabinoid levels in other peripheral organs or brain regions.” (DiPatrizio et al., 2011)
I’m reproducing a chart from Part 1, as a reminder of the complexity of the ECS (Matias et al., 2008 Figure 8).
We’ll keep this data in mind as we look at a study examining another type of gastric bypass surgery, laparoscopic sleeve gastrectomy (LSG) (Azar et al., 2019).
While noting:
“…Our study did not establish a causal relationship between circulating eCB levels and any of the metabolic/clinical parameters tested.” (Azar et al., 2019)
They observe:
“The present study is a 1-year prospective follow-up and covered a wide range of anthropometric, metabolic, and hepatic parameters collected in a uniform manner…. LSG surgery induces reductions in circulating 2-AG, AEA, and [Arachidonic acid (AA)] in humans, and these changes are correlated with clinical benefits related to the surgery.” (Azar et al., 2019)
Emphasis mine. Many of the papers they cite examine RYGB, including some of those discussed here. They note a variety of outcomes from these surgeries as far as circulating amounts of endocannabinoids go, and note a variety of possible confounders. One they don’t explore (amazingly) is diet, although they conclude:
“These findings also support the notion that therapeutic strategies aiming to decrease [endocannabinoid (eCB)] ‘tone’ (either by reducing eCBs or blocking the CB1R in periphery) in obese individuals may provide a clinically relevant tool to combat the obesity epidemic worldwide.” (Azar et al., 2019)
So despite some divergent findings in the literature (noted in the text) they conclude that there’s good reason to think there’s a mechanistic connection. Given the complexity of the ECS, divergent findings shouldn’t be a surprise, especially when a major variable like diet is often not even accounted for.
One effect that does seem to be pretty consistent is an increased Resting Energy Expenditure (REE), when accounting for Body Weight (BW):
“Notably, an excessive decline in REE is a major determinant of weight regain, and increased REE/BW is a strong predictor of excess weight loss after [bariatric surgery (BS)]” (K. Li et al., 2019).
Now, if you weight 300 lbs, and you lose 50% of your body weight after RYGB, your REE is going to go down, this is normal. But your REE/BW should go up, as a reduction of REE during weight loss is a sign of the “starvation effect” that several previous studies have examined, (Fothergill et al., 2016) one is one of the best:
“However, the classic Minnesota semistarvation experiment demonstrated a sustained suppression of RMR [Resting Metabolic Rate, a synonym for REE] during a period of weight regain with controlled refeeding when subjects were prevented from eating above baseline levels.” (Fothergill et al., 2016)
We’ll get into what might lower your metabolic rate pathologically in the HNE section, but for now we’ll just note that an increased REE/BW is a sign of ‘healthy’ weight loss—considering it’s a very risky surgery.
“There was a significant increase in REE/BW after RYGB and other types of BS…” (K. Li et al., 2019)
This was a meta-analysis of BS studies, and it has a number of issues, most notably that it doesn’t account for failed procedures, which are high in BS.
Finally we get into an actual exploration of the mechanism behind this surgery. And they happily include a nice ‘graphical abstract’ (Ye et al., 2020)
These authors actually compare what happens when you take rimonabant (the “CB1 inverse agonist” in the abstract) to RYGB. And it’s an astonishing study, they manage to resolve an number of questions previous authors have left open.
“RYGB and SG are the two most commonly performed bariatric procedures throughout the world. Some studies, however, suggested that RYGB has several advantages over SG in long term weight maintenance.” (Ye et al., 2020)
Here are the comparisons they performed:
1. RYGB to Sleeve Gastrectomy (SG).
2. Splanchnic denervation (SpDNV) of both RYGB and rimonabant animals.
3. Rimonabant to RYGB.
4. RYGB with and without added endocannabinoids.
What did they find? (In reverse order…)
4. Added endocannabinoids to RYGB animals: feeding AEA to RYGB animals caused a reversal of the key effect of the surgery. Sadly they didn’t use 2-AG also—they also note they did not measure the endocannabinoids the gut or other compartments:
“Conversely, administration of CB1 agonist [AEA] significantly attenuates the weight loss and energy-regulating effects of RYGB….
“The RYGB-AEA group gained more weight than the RYGB-placebo, while the sham-treated groups showed no significant difference (Figure 7C). There was no significant effect of AEA on food intake, but the RYGB-induced reduction in feeding efficiency was lost in the AEA-treated group (Figure 7D).” (Ye et al., 2020)
3. Rimonabant administration had similar effects to RYGB:
“Three-week treatment of DIO mice with rimonabant… caused a reduction in food intake that lasted for about a week…. The weight reducing action of [rimonabant] lasted for up to 18 days (Figure 6D). A PF [pair feeding] experiment showed that the anorectic effect of [rimonabant] does not entirely explain the weight loss evoked by such treatment… indicating that increase in energy expenditure may have contributed to its weight-reducing effects. Consistent with such possibility, a decrease in feeding efficiency, indicative of increased energy expenditure, was noted in the DIO mice treated with [rimonabant]...” (Ye et al., 2020)
This increase in metabolism has been shown before, by (Zhang et al., 2012), among others.
“Furthermore, RYGB induces a significant reduction in feeding efficiency, defined as weight gained per energy consumed (Figure S1D). However, the slight increase in fecal caloric loss (~1 kcal/day) observed in RYGB-operated mice… did not correlate with the amount of total body weight loss…. On the other hand, total energy expenditure… increases at post-week 3 after RYGB… despite a decrease in serum leptin concentration…. This suggests that weight loss post-RYGB is likely due to a primary central process that resets a new energy balance target, as previously suggested...” (Ye et al., 2020)
This change in energy expenditure is not seen in SG.
2. They also compared different nerves, to determine which nerves are actually controlling this process, adding new information not understood in (DiPatrizio et al., 2011; Han et al., 2018), and explaining a finding from (Guijarro et al., 2007): “Also, our data suggest an important role of the vagus in the outcome of RYGB surgery…” and a comment from (Borg et al., 2006) above.
Both rimonabant and RYGB stimulate activity in the splanchnic nerve, activity of which had not been explored in the studies I’ve cited previously. And, most remarkably, severing the splanchnic nerve (SpDNV in the text) eliminates the weight benefit of both rimonabant and RYGB (See RYGB+SpDNV vs Sham+SpDNV in figure 4).
“It is, however, intriguing that the loss of a large and crucial nerve such as the vagus had minimal consequences on the metabolic effects of RYGB, whereas the loss of the splanchnic had such dramatic effects.
“This anorectic effect of rimonabant was significantly mitigated after splanchnic denervation [SpDNV], again suggesting that intestinal CB1 is a key regulator of afferent energy signals along the gut-brain axis.” (Ye et al., 2020)
1. The critical difference between RYGB and SG, given the above, is that:
“However, level of Cb1 mRNA and protein expression were decreased in the Roux limb (jejunum) of RYGB-, but not SG-operated, mice…
“Together, these findings suggest that RYGB, but not SG, increases total energy expenditure, specifically RMR, by downregulating intestinal CB1 expression, leading to activation of the splanchnic sympathetic nerve traffic to induce browning of vWAT.” (Ye et al., 2020)
Calling (Ye et al., 2020) “groundbreaking”, (Hankir, 2020) is an extensive commentary on the study, finding, “The findings of Ye et al…. offer an unprecedented level of mechanistic insight into how RYGB produces such striking results on body weight and overall metabolic health.”
The authors examine many aspects of both the surgeries and the drugs, but the key finding is that the benefit of RYGB, like rimonabant, is due to its effect on the ECS.
Conclusion
So not only is the endocannabinoid system controlling in obesity, but it also seems to control in weight-loss surgery. While a rodent model might not perfectly describe the human experience, the use of rimonabant to prove the mechanism ties it into the human experience nicely, as do the use of human medical foods that are prescribed to induce weight gain.
There are many questions about how these surgeries fail. Obviously there is room in the surgery for a variant resection of the nervous system, but also the follow-up diet plays a big role in the success or failure.
Unfortunately these studies on surgery don’t really provide much insight into diet. The researchers all know which diets induce obesity most effectively, and they all use the same, or similar formulations.
One of the open questions is what causes increased feed efficiency, and why does the metabolic rate increase after a successful RYGB surgery? For answers to that, we need to get even further down into the weeds.
4-Hydroxynonenal (HNE)
HNE (also referred to as 4-HNE) is a product of the peroxidation of omega-6 (n-6) fats, exclusively. For this reason it is a very useful marker of pathological processes involving n-6 fats. The biggest source of n-6 in a modern diet is of course from industrial seed oils, and since the primary fat therein (linoleic acid, LA) is not produced endogenously, this allows one to map diet to outcome pretty effectively (Papastergiadis et al., 2014).
HNE Induces Obesity
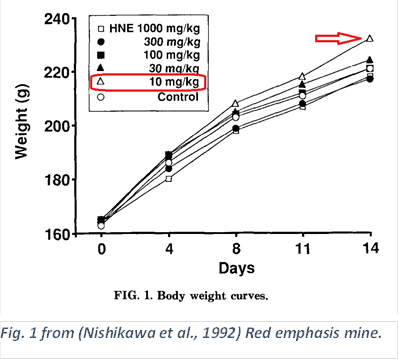
This was discovered inadvertently; HNE is quite toxic, and it was found in the course of a toxicology study on the effects of HNE on rats. As is often done, they started with an amount of the toxin in the neighborhood of physiological doses, and increase, typically until half of the animals die.
“Our study also shows that low doses of HNE appeared to increase body weight gain…” (Nishikawa et al., 1992)
The control used was corn oil, to which HNE was added.
HNE has been shown to be absorbed directly from the diet in animals (Kanazawa & Ashida, 1998; Paglialunga et al., 2015) and humans (Mubiru et al., 2017; Papastergiadis et al., 2014; Russell et al., 2003) but is also produced endogenously from seed oils consumed in the diet.
In human women, varying dietary intake of seed oils had a massive impact on DNA damage from HNE (Nair et al., 1997)
In 2001, the discoverer of HNE, Hermann Esterbauer, did an in vitro experiment using yeast cells. Similar to the previous study, HNE was added: “The remarkable differences from control cells are emphasized by the accumulation of lipid particles [see red circles in Fig. 2] and a fragmentation of the vacuole…” (Wonisch et al., 2001)
HNE is detoxified in the body by two endogenous antioxidants, glutathione (GSH) and aldehyde dehydrogenase (ALDH), and this system appears to be conserved, that is, active across a wide variety of species. (Singh, Niemczyk, Saini, et al., 2008).
“4-HNE specifically is metabolized (detoxified) by being reduced to an alcohol (aldehyde reductase), oxidized to a carboxylic acid (aldehyde dehydrogenase), or conjugated to glutathione (glutathione S-transferase) (10 –12). However, some fraction of 4-HNE escapes metabolism and forms adducts with other nucleophiles including both protein and DNA. In the case of protein, 4-HNE reacts avidly with the side chains of cysteine and histidine residues as well as lysine” (Grimsrud et al., 2007)
Building upon the findings of (Wonisch et al., 2001), (Singh, Niemczyk, Saini, et al., 2008) induced a “knock-out” (KO), a genetic mutation, of one of the enzymes involved in GSH metabolism (mGSTA4-4) in two strains of mice. In one strain, this induced obesity, in the other, something protected the mice.
“Regardless of the underlying mechanism, the observed strain difference was fortuitous as it established that the level of 4-HNE correlates with malonyl-CoA levels and with fat accumulation.”
“…Our work in conjunction with published results by others suggests the existence of a positive feedback loop involving 4-HNE and perpetuating obesity as long as food is available.”
They note that either the KO, or diet-induced obesity (DIO) as performed in (Grimsrud et al., 2007) can induce this process. Grimsrud et al., used the earlier version (Bio-Serv 1850) of the Surwit diet (Bio-Serv, 2015; Research Diets, Inc., 2006a; Surwit et al., 1988). This will become helpful later…
HNE Induction of Obesity is “Universal”
(Singh, Niemczyk, Zimniak, et al., 2008) continued the work of (Singh, Niemczyk, Saini, et al., 2008) in rodents with a paper looking at the roundworm, Caenorhabditis elegans (C. elegans), a standard model for working out fundamental metabolic processes.
“We have previously shown that genetically modified mice with an elevated level of the lipid peroxidation product 4‐HNE become obese. We now demonstrate that the process is phylogenetically conserved and thus likely to be universal.” (Singh, Niemczyk, Zimniak, et al., 2008)
They did this by treating the worms directly with HNE, and also by knocking out the genes for either GSH enzymes or ALDH.
“The increase in stored fat was similar for worms directly exposed to 4-HNE (Figure 5) and for those with silenced 4-HNE-metabolizing enzymes (Figure 2 and Figure 3), even though the former treatment was markedly more effective in raising the level of 4-HNE protein adducts (Figure 4 versus Figure 1).”
“Except for 4-HNE, there is no known common substrate of the two rather dissimilar C. elegans enzymes used in the present work, i.e., the products of gst-10 [GSH] and alh-1 [ALDH] genes. Moreover, 4-HNE is the only known common substrate of the above-mentioned two enzymes and the murine mGSTA4-4.” (Singh, Niemczyk, Zimniak, et al., 2008)
Additionally, they used a strain of worm overexpressing glutathione enzymes (Ayyadevara et al., 2005) to determine the inverse effect of HNE on obesity, and indeed these worms exhibited lower fat accumulation than the control (Singh, Niemczyk, Zimniak, et al., 2008).
What appears to be happening is that HNE is altering the Randle Cycle (Hue & Taegtmeyer, 2009; Randle et al., 1963), a basic process by which the cell determines what fuel it should be using, and what it should be doing with fuels not in use.
“Malonyl-CoA has a dual metabolic function: it is the substrate for fatty acid synthesis, and it prevents fatty acid β-oxidation [reviewed in ref. 36]. Therefore, elevated malonyl-CoA levels lead to an accumulation of fatty acids and, consequently, of triglycerides. In fact, experimental lowering of malonyl-CoA levels results in a lean phenotype in mammals [37-39] as well as in C. elegans [40].”
“In turn, elevated 4-HNE realigns metabolism to favor fat deposition by increasing malonyl-CoA production, as described by us in the present report.
“We propose that these two partial processes – fat-induced 4-HNE generation and 4-HNE-triggered fat accumulation form a positive feedback loop that perpetuates lipid storage as long as food is available.” (Singh, Niemczyk, Zimniak, et al., 2008)
And, of course, as long as HNE is being generated.
The Most Common Human Mutation
Deficiency in aldehyde dehydrogenase enzymes, one of the KO’s performed in C. elegans above, is likely the most common human genetic mutation (Chen et al., 2020). The ALDH2*2 variant is responsible for the “Asian flush” reaction to alcohol consumption, due to the inability to detoxify acetaldehyde, the major metabolite of alcohol in the body.
“In addition to acetaldehyde, ALDH2 also metabolizes various bioactive toxic aldehydes, including acrolein, malondialdehyde and 4-hydroxynonenal (4-HNE).”
“…Importantly, several large-scale meta-analyses of genome-wide association studies revealed that this inactivating mutation is strongly associated with type 2 diabetes, body mass index, and serum lipids in East Asians. A validation study further confirmed a close association of ALDH2*2 with visceral fat distribution in 2,958 Chinese subjects.” (Chang et al., 2020)
These authors took the work in rats and roundworms above to its logical conclusion: a human KO mutation thought to correspond to many disease associated with excess levels of toxic aldehydes. They performed a “Knock-In” (KI, they added the human gene to these mice) of the human ALDH2*2 gene to see if the human mutation has a similar effect to the KO models used above. Additionally, they used the D12331 version of the Surwit diet to induce obesity; as mentioned above, a common model (Bio-Serv, 2015; Research Diets, Inc., 2006a; Surwit et al., 1988).
It’s important to note that the negative effect of this mutation on the mice was much smaller than the negative effect of the diet. As ALDH is only a partial protection from toxic aldehydes, this is not surprising.
The authors found:
ALDH2*2 KI decreased detoxification of HNE, shown by increased protein damage due to HNE adduction.
HNE protein damage reduced fatty-acid metabolism (fatty acid oxidation, FAO), energy expenditure, and adaptive thermogenesis through damage to mitochondrial electron transport chain.
ALDH2*2 KI worsened the effect of Surwit diet due to the damage from HNE. “These data indicated Aldh2KI mice were prone to diet-induced obesity due to reduced energy expenditure but not intake.”
A pharmacological stimulant of ALDH, AD-9308, reduced HNE levels and adiposity, in WT and KI mice. (Several authors have a financial interest in the success of AD-9308.)
“Furthermore, addition of 4-HNE decreased FAO rate of induced primary brown adipocytes isolated from WT mice in a dose-dependent manner; FAO rates were reduced by 36% (P < 0.01) and 60% at 5 and10 μM4-HNE, respectively (P < 0.01)(Fig. 3h). …we found a significant reduction in the enzymatic activity of mitochondrial respiratory complexes I, II, and III, but not complex IV in Aldh2KI mice compared with WT mice (Fig 3i).” (Chang et al., 2020)
AD-9308 dose-dependently reduced the effect of the Surwit diet on both strains of mice.
Fatty Acid Metabolism Modulates HNE Toxicity
A ketogenic diet is a well-recognized therapeutic diet for several conditions including epilepsy and weight loss (Hallberg et al., 2018; Kossoff et al., 2018). Unlike the ketogenic diet prescribed to humans for diabetes and obesity, which generally eschews seed oils (Phinney & Virta, 2020), the model used in rodent experiments (F3666 is used in every study in this section) is comprised of a good portion of seed oils—via lard and corn oil (Lecker, 2011).
Oddly, while a high-fat diet induces obesity as we have discussed at length, a higher-fat ketogenic diet does not, even when it contains ample LA:
“It is of particular interest that the specific effect of moderately high fat diet (HFD) to alter hypothalamic proliferative remodelling is not shared by ultra high fat ketogenic diet (KD). While this could be due to the difference in the source of dietary fat, in both diets fat is predominantly of animal origin (lard and/or butterfat). This may point to a specific requirement for the presence of both fat and carbohydrate in the diet, although a protective effect of ketosis masking the effect of dietary fat in the case of KD cannot be excluded.” (McNay & Speakman, 2013)
The demonstrated ability of HNE to induce obesity provides an explanation for this phenomenon. The massive upregulation of FAO in a ketogenic diet creates an additional disposal pathway for HNE: it is burned as fuel.
“Our results showed that livers from rats fed ketogenic diet or high fat mix diet had high ω-6 [omega-6] polyunsaturated fatty acid concentrations and markers of oxidative stress. However, high concentrations of HNE (1.6 ± 0.5 nmol/g) and ONE (0.9 ± 0.2 nmol/g) were only found in livers from rats fed the high fat mix diet. Livers from rats fed the ketogenic diet had low HNE (0.8 ± 0.1 nmol/g) and ONE (0.4 ± 0.07 nmol/g), similar to rats fed the standard diet” (Q. Li et al., 2012).
And this protected them from HNE’s ability to addle the Randle Cycle, tipping it towards over-production of malonyl-CoA and thus triglyceride over-production:
“A possible explanation is that the predominant pathway of HNE catabolism (i.e. beta oxidation) is activated in the liver by the ketogenic diet. This is consistent with a 10 fold decrease in malonyl-CoA in livers from rats fed a ketogenic diet compared to a standard diet” (Q. Li et al., 2012).
Additionally, as expected given the above, redox status is improved and thiols (lipoic acid and glutathione) are increased by the ketogenic F3666 diet (Jarrett et al., 2008): thiols are the primary detoxification system for HNE.
Whence Feed Efficiency?
Several of the studies discussed have mentioned the concept of “feed efficiency” (A. Alvheim et al., 2014; A. R. Alvheim et al., 2012, 2012, 2013; Ghosh et al., 2019; Osei-Hyiaman et al., 2005; Ravinet Trillou et al., 2004; Witkamp, 2018), which is defined as how effective a given diet is in adding body weight to an animal. This is a very important concept in agriculture, where narrow margins make foods that cause animals to grow more quickly a valuable commodity.
These papers (Chang et al., 2020; Q. Li et al., 2012; McNay & Speakman, 2013) make clear that, while LA via HNE is a central part of the phenomenon of feed efficiency, there are multiple factors that play a role, especially carbohydrate consumption—specifically explored in (Ghosh et al., 2019).
Both RYGB and ECS inhibition succeed because they decrease feed efficiency (Guijarro et al., 2007, 2008; Witkamp, 2018; Ye et al., 2020; Zhang et al., 2012) even more so than there effects on caloric intake.
Conclusion
The modulation of obesity via ALDH detoxification of HNE in (Chang et al., 2020) in a diet which does not contain a large amount of HNE precursor LA suggests that this may be a fundamental pathway underlying the obesogenic effect of all experimental and human diets, it is, “phylogenetically conserved and thus likely to be universal” (Singh, Niemczyk, Zimniak, et al., 2008). The fact that HNE is derived exclusively from n-6 fats, and primarily from LA, allows us to conclude that this is likely a major pathway behind our obesity pandemic.
Reconciling RYGB and HNE
Prior to weight loss, RYGB induces:
Increased fatty acid oxidation. (K. Li et al., 2019)
Decreased oxidative stress. (Tozzo et al., 2020)
Increased energy expenditure. (K. Li et al., 2019)
Rimonabant, an endocannabinoid system inhibitor, also has these effects (Osei-Hyiaman et al., 2008), while also directly upregulating glutathione production (Jbilo et al., 2005).
JD5037, a peripheral ECS inhibitor (it does not affect the brain) with similar effects to rimonabant, directly reduces HNE levels in the liver and white adipose tissue of mice (Liu et al., 2019).
Additionally, pharmacologically upregulating the ECS abrogates the benefits of RYGB on metabolism.
Thus the effects of both rimonabant (and other ECS inhibitors) and RYGB depend on a common pathway, and are likely both dependent on the modulation of HNE for their effect on obesity.
Conclusion
The rapid increases in obesity and the associated diseases demands an explanation.
It seems that a cause of these diseases must meet the following criteria:
1. Pan-Species
This is a problem that we see in all human populations, and the animal species that associate with them, which includes pets and lab animals but also feral animals like rats, pigeons, and racoons (Katsnelson 2010).
This eliminates genetic as primary causes, and virtually all pathogens, which do not cross species lines easily.
2. Diet-based
While many environmental factors could conceivably have an impact, that fact that pandemic obesity is occurring in all environments suggests that environmental effects other than diet are minor. We have much evidence in humans that even dietary changes in the short term in groups that have not changed their environment significantly are subject to drastic changes in health within a single generation.
3. Novel
Must be a food that was recently introduced, contemporaneously with the pandemics of chronic diseases including T2DM and obesity
4. Mechanistic confirmation
Epidemiological data such as the above has too often mislead. We require a solid base of mechanistic data that shows positive and negative confirmation of effects in animals and humans. Confounders need to be accounted for.
5. Contained in D12492 and Ensure
It must be included, of course, in the most commonly-used obesogenic diet in the world, and, additionally, in the medical foods that are prescribed to humans to induce weight gain.
Linoleic acid, via industrially-produced seed oils, is the only item that meets the above criteria.
Perhaps the most compelling evidence to emerge from this process is the fact that Ensure and Boost are both used clinically by physicians to make humans gain weight, by bench scientists with lab animals to induce obesity, and have equivalent ingredients and actions to the cookie-dough diet, D12492 or the newer D12451 (DiPatrizio et al., 2011; Meguid et al., 2004; Shin et al., 2011). Seed oils, sugar, and refined carbohydrates are basically the definition of junk food, and this has been recognized going back to the 1930s. And even physicians, although they don’t seem to realize what they are doing, recognize that feeding junk food of that composition to Grandma will make her fat.
I think it’s safe to say, while there are certainly confounders, that we can add obesity to the list of negative effects that seed oils have on humans.
References
Abbott Laboratories, Inc. (2020, November 9). Ensure® Original Vanilla Meal Replacement Shake [Advertisement]. Ensure®: Strength and Energy. https://ensure.com/nutrition-products/ensure-original/vanilla-shake
Alvheim, A. R., Malde, M. K., Osei‐Hyiaman, D., Hong, Y. H., Pawlosky, R. J., Madsen, L., Kristiansen, K., Frøyland, L., & Hibbeln, J. R. (2012). Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity, 20(10), 1984–1994. https://doi.org/10.1038/oby.2012.38
Alvheim, A. R., Torstensen, B. E., Lin, Y. H., Lillefosse, H. H., Lock, E.-J., Madsen, L., Hibbeln, J. R., & Malde, M. K. (2013). Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice. The British Journal of Nutrition, 109(8), 1508–1517. https://doi.org/10.1017/S0007114512003364
Alvheim, A., Torstensen, B. E., Lin, Y. H., Lillefosse, H. H., Lock, E.-J., Madsen, L., Frøyland, L., Hibbeln, J. R., & Malde, M. K. (2014). Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids, 49(1), 59–69. https://doi.org/10.1007/s11745-013-3842-y
Arnone, M., Maruani, J., Chaperon, F., Thiébot, M. H., Poncelet, M., Soubrié, P., & Le Fur, G. (1997). Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology, 132(1), 104–106. https://doi.org/10.1007/s002130050326
Avalos, B., Argueta, D. A., Perez, P. A., Wiley, M., Wood, C., & DiPatrizio, N. V. (2020). Cannabinoid CB1 Receptors in the Intestinal Epithelium Are Required for Acute Western-Diet Preferences in Mice. Nutrients, 12(9). https://doi.org/10.3390/nu12092874
Ayyadevara, S., Engle, M. R., Singh, S. P., Dandapat, A., Lichti, C. F., Benes, H., Shmookler Reis, R. J., Liebau, E., & Zimniak, P. (2005). Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell, 4(5), 257–271. https://doi.org/10.1111/j.1474-9726.2005.00168.x
Azar, S., Sherf-Dagan, S., Nemirovski, A., Webb, M., Raziel, A., Keidar, A., Goitein, D., Sakran, N., Shibolet, O., Tam, J., & Zelber-Sagi, S. (2019). Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obesity Surgery, 29(1), 268–276. https://doi.org/10.1007/s11695-018-3517-0
Bio-Serv. (2015). Mouse Diet, High Fat, Fat Calories (60%), Paste (1850) [Advertisement]. Bio-Serv. https://www.bio-serv.com/product/HFPaste.html
BOOST Plus®. (2021). [Advertisement]. BOOST®.
https://www.boost.com
Borg, C. M., le Roux, C. W., Ghatei, M. A., Bloom, S. R., Patel, A. G., & Aylwin, S. J. B. (2006). Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. The British Journal of Surgery, 93(2), 210–215. https://doi.org/10.1002/bjs.5227
Castaneda, D., Popov, V. B., Wander, P., & Thompson, C. C. (2019). Risk of Suicide and Self-harm Is Increased After Bariatric Surgery-a Systematic Review and Meta-analysis. Obesity Surgery, 29(1), 322–333. https://doi.org/10.1007/s11695-018-3493-4
Chang, Y.-C., Lee, H.-L., Yang, W., Hsieh, M.-L., Liu, C.-C., Lee, T.-Y., Huang, J.-Y., Nong, J.-Y., Li, F.-A., Chuang, H.-L., Ding, Z.-Z., Su, W.-L., Chueh, L.-Y., Tsai, Y.-T., Chen, C.-H., Mochly-Rosen, D., & Chuang, L.-M. (2020). A common East Asian-specific ALDH2 mutation causes obesity and insulin resistance: Therapeutic effect of reducing toxic aldehydes by ALDH2 activation. https://doi.org/10.21203/rs.3.rs-104384/v1
Chen, C.-H., Ferreira, J. C. B., Joshi, A. U., Stevens, M. C., Li, S.-J., Hsu, J. H.-M., Maclean, R., Ferreira, N. D., Cervantes, P. R., Martinez, D. D., Barrientos, F. L., Quintanares, G. H. R., & Mochly-Rosen, D. (2020). Novel and prevalent non-East Asian ALDH2 variants; Implications for global susceptibility to aldehydes’ toxicity. EBioMedicine, 55, 102753. https://doi.org/10.1016/j.ebiom.2020.102753
DiPatrizio, N. V., Astarita, G., Schwartz, G., Li, X., & Piomelli, D. (2011). Endocannabinoid signal in the gut controls dietary fat intake. Proceedings of the National Academy of Sciences of the United States of America, 108(31), 12904–12908. https://doi.org/10.1073/pnas.1104675108
Duarte, C., Alonso, R., Bichet, N., Cohen, C., Soubrié, P., & Thiébot, M.-H. (2004). Blockade by the Cannabinoid CB1 Receptor Antagonist, Rimonabant (SR141716), of the Potentiation by Quinelorane of Food-Primed Reinstatement of Food-Seeking Behavior. Neuropsychopharmacology, 29(5), 911–920. https://doi.org/10.1038/sj.npp.1300370
Ensure vs. Boost: Which Is Healthier? (2020, August 26). Healthline. https://www.healthline.com/nutrition/ensure-vs-boost
Faria, G. R. (2017). A brief history of bariatric surgery. Porto Biomedical Journal, 2(3), 90–92. https://doi.org/10.1016/j.pbj.2017.01.008
Fothergill, E., Guo, J., Howard, L., Kerns, J. C., Knuth, N. D., Brychta, R., Chen, K. Y., Skarulis, M. C., Walter, M., Walter, P. J., & Hall, K. D. (2016). Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity, 24(8), 1612–1619. https://doi.org/10.1002/oby.21538
Ghosh, S., O’Connell, J. F., Carlson, O. D., González‐Mariscal, I., Kim, Y., Moaddel, R., Ghosh, P., & Egan, J. M. (2019). Linoleic acid in diets of mice increases total endocannabinoid levels in bowel and liver: Modification by dietary glucose. Obesity Science & Practice, 5(4), 383–394. https://doi.org/10.1002/osp4.344
Grimsrud, P. A., Picklo, M. J., Griffin, T. J., & Bernlohr, D. A. (2007). Carbonylation of Adipose Proteins in Obesity and Insulin Resistance: Identification of Adipocyte Fatty Acid-binding Protein as a Cellular Target of 4-Hydroxynonenal*. Molecular & Cellular Proteomics, 6(4), 624–637. https://doi.org/10.1074/mcp.M600120-MCP200
Guijarro, A., Kirchner, H., & Meguid, M. M. (2006). Catabolic effects of gastric bypass in a diet-induced obese rat model. Current Opinion in Clinical Nutrition and Metabolic Care, 9(4), 423–435. https://doi.org/10.1097/01.mco.0000232903.04910.7b
Guijarro, A., Osei-Hyiaman, D., Harvey-White, J., Kunos, G., Suzuki, S., Nadtochiy, S., Brookes, P. S., & Meguid, M. M. (2008). Sustained Weight Loss After Roux-en-Y Gastric Bypass Is Characterized by Down Regulation of Endocannabinoids and Mitochondrial Function. Annals of Surgery, 247(5), 779–790. https://doi.org/10.1097/SLA.0b013e318166fd5f
Guijarro, A., Suzuki, S., Chen, C., Kirchner, H., Middleton, F. A., Nadtochiy, S., Brookes, P. S., Niijima, A., Inui, A., & Meguid, M. M. (2007). Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293(4), R1474-1489. https://doi.org/10.1152/ajpregu.00171.2007
Hallberg, S. J., McKenzie, A. L., Williams, P. T., Bhanpuri, N. H., Peters, A. L., Campbell, W. W., Hazbun, T. L., Volk, B. M., McCarter, J. P., Phinney, S. D., & Volek, J. S. (2018). Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Therapy, 9(2), 583–612. https://doi.org/10.1007/s13300-018-0373-9
Han, W., Tellez, L. A., Perkins, M. H., Perez, I. O., Qu, T., Ferreira, J., Ferreira, T. L., Quinn, D., Liu, Z.-W., Gao, X.-B., Kaelberer, M. M., Bohórquez, D. V., Shammah-Lagnado, S. J., Lartigue, G. de, & Araujo, I. E. de. (2018). A Neural Circuit for Gut-Induced Reward. Cell, 175(3), 665-678.e23. https://doi.org/10.1016/j.cell.2018.08.049
Hankir, M. K. (2020). A sympathetic gut connection drives the metabolic benefits of Roux-en-Y gastric bypass. Cell Stress, 4(12), 265. https://doi.org/10.15698/cst2020.12.236
Hansen, T. T., Jakobsen, T. A., Nielsen, M. S., Sjödin, A., Le Roux, C. W., & Schmidt, J. B. (2016). Hedonic Changes in Food Choices Following Roux-en-Y Gastric Bypass. Obesity Surgery, 26(8), 1946–1955. https://doi.org/10.1007/s11695-016-2217-x
Harvey, E. J., Arroyo, K., Korner, J., & Inabnet, W. B. (2010). Hormone Changes Affecting Energy Homeostasis after Metabolic Surgery. The Mount Sinai Journal of Medicine, New York, 77(5), 446–465. https://doi.org/10.1002/msj.20203
Hickey, M. S., Pories, W. J., MacDonald, K. G., Cory, K. A., Dohm, G. L., Swanson, M. S., Israel, R. G., Barakat, H. A., Considine, R. V., Caro, J. F., & Houmard, J. A. (1998). A new paradigm for type 2 diabetes mellitus: Could it be a disease of the foregut? Annals of Surgery, 227(5), 637–643; discussion 643-644. https://doi.org/10.1097/00000658-199805000-00004
Higuchi, S., Irie, K., Yamaguchi, R., Katsuki, M., Araki, M., Ohji, M., Hayakawa, K., Mishima, S., Akitake, Y., Matsuyama, K., Mishima, K., Mishima, K., Iwasaki, K., & Fujiwara, M. (2012). Hypothalamic 2-Arachidonoylglycerol Regulates Multistage Process of High-Fat Diet Preferences. PLOS ONE, 7(6), e38609. https://doi.org/10.1371/journal.pone.0038609
Hue, L., & Taegtmeyer, H. (2009). The Randle cycle revisited: A new head for an old hat. American Journal of Physiology-Endocrinology and Metabolism, 297(3), E578–E591. https://doi.org/10.1152/ajpendo.00093.2009
Jarrett, S. G., Milder, J. B., Liang, L.-P., & Patel, M. (2008). The ketogenic diet increases mitochondrial glutathione levels. Journal of Neurochemistry, 106(3), 1044–1051. https://doi.org/10.1111/j.1471-4159.2008.05460.x
Jbilo, O., Ravinet‐Trillou, C., Arnone, M., Buisson, I., Bribes, E., Péleraux, A., Pénarier, G., Soubrié, P., Fur, G. L., Galiègue, S., & Casellas, P. (2005). The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. The FASEB Journal, 19(11), 1567–1569. https://doi.org/10.1096/fj.04-3177fje
Kanazawa, K., & Ashida, H. (1998). Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochimica Et Biophysica Acta, 1393(2–3), 349–361. https://doi.org/10.1016/s0005-2760(98)00089-7
Katsnelson, A. (2010). Lab Animals and Pets Face Obesity Epidemic. Nature. https://doi.org/10.1038/news.2010.628
Koch, J. E. (2001). Δ9-THC stimulates food intake in Lewis rats: Effects on chow, high-fat and sweet high-fat diets. Pharmacology, Biochemistry, and Behavior, 68(3), 539–543. https://doi.org/10.1016/s0091-3057(01)00467-1
Kossoff, E. H., Zupec‐Kania, B. A., Auvin, S., Ballaban‐Gil, K. R., Christina Bergqvist, A. G., Blackford, R., Buchhalter, J. R., Caraballo, R. H., Cross, J. H., Dahlin, M. G., Donner, E. J., Guzel, O., Jehle, R. S., Klepper, J., Kang, H., Lambrechts, D. A., Liu, Y. M. C., Nathan, J. K., Nordli, D. R., … Wirrell, E. C. (2018). Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open, 3(2), 175–192. https://doi.org/10.1002/epi4.12225
Lecker, J. (2011, January). Ketogenic Diet F3666 [Advertisement]. Bio-Serv. https://www.bio-serv.com/product/Ketogenic_Diet.html
Li, K., Shi, W., Zhao, F., Yang, C., Dai, Q., Wang, B., & Li, Y. (2019). Changes in Energy Expenditure of Patients with Obesity Following Bariatric Surgery: A Systematic Review of Prospective Studies and Meta-analysis. Obesity Surgery, 29(7), 2318–2337. https://doi.org/10.1007/s11695-019-03851-2
Li, Q., Tomcik, K., Zhang, S., Puchowicz, M. A., & Zhang, G.-F. (2012). Dietary-regulation of catabolic disposal of 4-hydroxynonenal analogs in rat liver. Free Radical Biology & Medicine, 52(6), 1043–1053. https://doi.org/10.1016/j.freeradbiomed.2011.12.022
Liu, J., Godlewski, G., Jourdan, T., Liu, Z., Cinar, R., Xiong, K., & Kunos, G. (2019). Cannabinoid-1 Receptor Antagonism Improves Glycemic Control and Increases Energy Expenditure Through Sirtuin-1/Mechanistic Target of Rapamycin Complex 2 and 5′Adenosine Monophosphate–Activated Protein Kinase Signaling. Hepatology, 69(4), 1535–1548. https://doi.org/10.1002/hep.30364
Matias, I., Petrosino, S., Racioppi, A., Capasso, R., Izzo, A. A., & Di Marzo, V. (2008). Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Molecular and Cellular Endocrinology, 286(1-2 Suppl 1), S66-78. https://doi.org/10.1016/j.mce.2008.01.026
McNay, D. E. G., & Speakman, J. R. (2013). High fat diet causes rebound weight gain. Molecular Metabolism, 2(2), 103–108. https://doi.org/10.1016/j.molmet.2012.10.003
Meguid, M. M., Ramos, E. J. B., Suzuki, S., Xu, Y., George, Z. M., Das, U. N., Hughes, K., Quinn, R., Chen, C., Marx, W., & Cunningham, P. R. G. (2004). A surgical rat model of human Roux-en-Y gastric bypass. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract, 8(5), 621–630. https://doi.org/10.1016/j.gassur.2004.02.003
Mubiru, E., Jacxsens, L., Papastergiadis, A., Lachat, C., Shrestha, K., Mozumder, N. H. M. R., & Meulenaer, B. D. (2017). Exposure assessment of epoxy fatty acids through consumption of specific foods available in Belgium. Food Additives & Contaminants: Part A, 34(6), 1000–1011. https://doi.org/10.1080/19440049.2017.1310399
Nair, J., Vaca, C. E., Velic, I., Mutanen, M., Valsta, L. M., & Bartsch, H. (1997). High dietary omega-6 polyunsaturated fatty acids drastically increase the formation of etheno-DNA base adducts in white blood cells of female subjects. Cancer Epidemiology and Prevention Biomarkers, 6(8), 597–601. https://cebp.aacrjournals.org/content/6/8/597
Nishikawa, A., Sodum, R., & Chung, F.-L. (1992). Acute toxicity of trans-4-hydroxy-2-nonenal in fisher 344 rats. Lipids, 27(1), 54–58. https://doi.org/10.1007/BF02537060
Osei-Hyiaman, D., DePetrillo, M., Pacher, P., Liu, J., Radaeva, S., Bátkai, S., Harvey-White, J., Mackie, K., Offertáler, L., Wang, L., & Kunos, G. (2005). Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. Journal of Clinical Investigation, 115(5), 1298–1305. https://doi.org/10.1172/JCI200523057
Osei-Hyiaman, D., Liu, J., Zhou, L., Godlewski, G., Harvey-White, J., Jeong, W., Bátkai, S., Marsicano, G., Lutz, B., Buettner, C., & Kunos, G. (2008). Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. Journal of Clinical Investigation, 118(9), 3160–3169. https://doi.org/10.1172/JCI34827
Paglialunga, S., Ludzki, A., Root-McCaig, J., & Holloway, G. P. (2015). In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia, 58(5), 1071–1080. https://doi.org/10.1007/s00125-015-3531-x
Papastergiadis, A., Fatouh, A., Jacxsens, L., Lachat, C., Shrestha, K., Daelman, J., Kolsteren, P., Van Langenhove, H., & De Meulenaer, B. (2014). Exposure assessment of Malondialdehyde, 4-Hydroxy-2-(E)-Nonenal and 4-Hydroxy-2-(E)-Hexenal through specific foods available in Belgium. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 73, 51–58. https://doi.org/10.1016/j.fct.2014.06.030
Phinney, S. D., & Virta, T. (2020, February 4). Which fats and oils should I eat on a ketogenic diet? [Advertisement]. Virta Health. https://virtahealth.webflow.io/faq/fats-oils-ketogenic-diet
Randle, P. J., Garland, P. B., Hales, C. N., & Newsholme, E. A. (1963). The Glucose Fatty-Acid Cycle Its Role In Insulin Sensitivity And The Metabolic Disturbances Of Diabetes Mellitus. The Lancet, 281(7285), 785–789. https://doi.org/10.1016/S0140-6736(63)91500-9
Ravinet Trillou, C., Delgorge, C., Menet, C., Arnone, M., & Soubrié, P. (2004). CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. International Journal of Obesity, 28(4), 640–648. https://doi.org/10.1038/sj.ijo.0802583
Research Diets, Inc. (2006a). D12331 Formula (Surwit)—OpenSource Diets. Research Diets, Inc. https://researchdiets.com/formulas/d12331
Research Diets, Inc. (2006b). D12492 Formula [Advertisement]. D12492. https://researchdiets.com/formulas/d12492
Rubino, F., Gagner, M., Gentileschi, P., Kini, S., Fukuyama, S., Feng, J., & Diamond, E. (2004). The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Annals of Surgery, 240(2), 236–242. https://doi.org/10.1097/01.sla.0000133117.12646.48
Russell, A. P., Gastaldi, G., Bobbioni-Harsch, E., Arboit, P., Gobelet, C., Dériaz, O., Golay, A., Witztum, J. L., & Giacobino, J.-P. (2003). Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: A case of good vs. bad lipids? FEBS Letters, 551(1–3), 104–106. https://doi.org/10.1016/s0014-5793(03)00875-5
Shin, A. C., Zheng, H., Pistell, P. J., & Berthoud, H.-R. (2011). Roux-en-Y gastric bypass surgery changes food reward in rats. International Journal of Obesity (2005), 35(5), 642–651. https://doi.org/10.1038/ijo.2010.174
Simiand, J., Keane, M., Keane, P. E., & Soubrié, P. (1998). SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behavioural Pharmacology, 9(2), 179–181. https://pubmed.ncbi.nlm.nih.gov/10065938/
Singh, S. P., Niemczyk, M., Saini, D., Awasthi, Y. C., Zimniak, L., & Zimniak, P. (2008). Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry, 47(12), 3900–3911. https://doi.org/10.1021/bi702124u
Singh, S. P., Niemczyk, M., Zimniak, L., & Zimniak, P. (2008). Fat accumulation in Caenorhabditis elegans triggered by the electrophilic lipid peroxidation product 4-Hydroxynonenal (4-HNE). Aging, 1(1), 68–80. https://doi.org/10.18632/aging.100005
Surwit, R. S., Kuhn, C. M., Cochrane, C., McCubbin, J. A., & Feinglos, M. N. (1988). Diet-induced type II diabetes in C57BL/6J mice. Diabetes, 37(9), 1163–1167. https://doi.org/10.2337/diab.37.9.1163
Tozzo, C., Moreira, E. A. M., de Freitas, M. B., da Silva, A. F., Portari, G. V., & Wilhelm Filho, D. (2020). Effect of RYGB on Oxidative Stress in Adults: A 6-Year Follow-up Study. Obesity Surgery, 30(9), 3301–3308. https://doi.org/10.1007/s11695-020-04561-w
Ullrich, J., Ernst, B., Wilms, B., Thurnheer, M., & Schultes, B. (2013). Roux-en Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obesity Surgery, 23(1), 50–55. https://doi.org/10.1007/s11695-012-0754-5
W O Griffen, J. (1977). A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Annals of Surgery, 186(4), 500. https://doi.org/10.1097/00000658-197710000-00012
Wickremesekera, K., Miller, G., Naotunne, T. D., Knowles, G., & Stubbs, R. S. (2005). Loss of Insulin Resistance after Roux-en-Y Gastric Bypass Surgery: A Time Course Study. Obesity Surgery, 15(4), 474–481. https://doi.org/10.1381/0960892053723402
Witkamp, R. F. (2018). The role of fatty acids and their endocannabinoid-like derivatives in the molecular regulation of appetite. Molecular Aspects of Medicine, 64, 45–67. https://doi.org/10.1016/j.mam.2018.01.002
Wonisch, W., Zellnig, G., Kohlwein, S. D., Schaur, R. J., Bilinski, T., Tatzber, F., & Esterbauer, H. (2001). Ultrastructural analysis of HNE-treated Saccharomyces cerevisiae cells reveals fragmentation of the vacuole and an accumulation of lipids in the cytosol. Cell Biochemistry and Function, 19(1), 59–64. https://doi.org/10.1002/cbf.888
Ye, Y., Abu El Haija, M., Morgan, D. A., Guo, D., Song, Y., Frank, A., Tian, L., Riedl, R. A., Burnett, C. M. L., Gao, Z., Zhu, Z., Shahi, S. K., Zarei, K., Couvelard, A., Poté, N., Ribeiro-Parenti, L., Bado, A., Noureddine, L., Bellizzi, A., … Mokadem, M. (2020). Endocannabinoid Receptor-1 and Sympathetic Nervous System Mediate the Beneficial Metabolic Effects of Gastric Bypass. Cell Reports, 33(4), 108270. https://doi.org/10.1016/j.celrep.2020.108270
Zhang, L.-N., Gamo, Y., Sinclair, R., Mitchell, S. E., Morgan, D. G., Clapham, J. C., & Speakman, J. R. (2012). Effects of Chronic Oral Rimonabant Administration on Energy Budgets of Diet-Induced Obese C57BL/6 Mice. Obesity, 20(5), 954–962. https://doi.org/10.1038/oby.2011.357